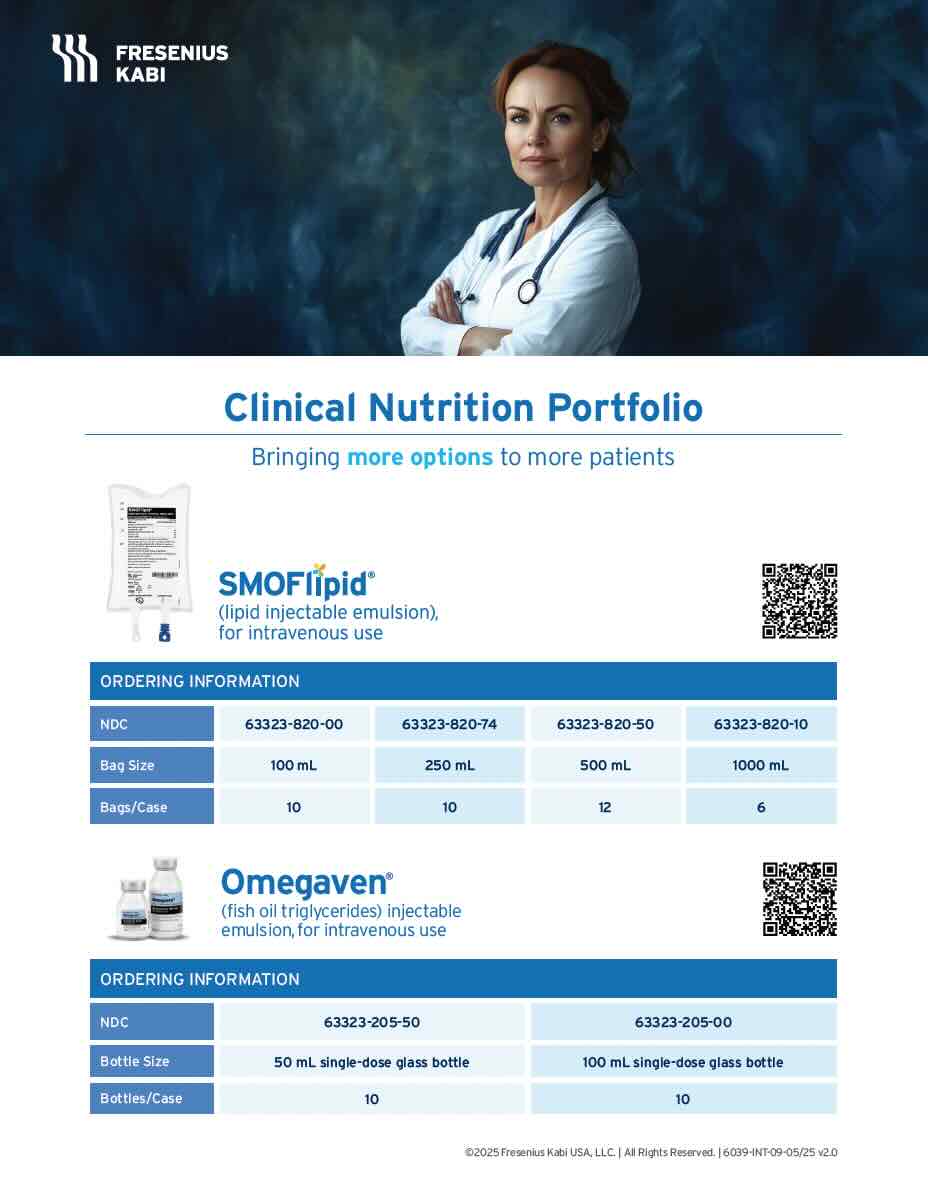
Bringing more to parenteral nutrition (PN)
As the US market leader in lipid injectable emulsions (ILEs),1 Fresenius Kabi offers more options, more choices, and more variety to nourish critically or chronically ill patients of any age—from hospital to home.
At the forefront of PN
A tireless dedication to driving advancements in lifesaving medicines and technologies has led to many firsts in the field, like introducing the first lipid PN product for adults in more than 40 years, keeping Fresenius Kabi at the forefront of PN.*
*Portfolio data on file.