

Pediatric parenteral nutrition (PN): more options for more young patients
PN has been administered to children for more than 40 years.1,2 This form of nutrition support can be lifesaving for many pediatric patients who are unable to receive adequate nutrition via the enteral route, which is often due to a gastrointestinal disease or condition such as intestinal failure, short bowel syndrome, or a bowel obstruction.3
As the US market leader in lipid injectable emulsions (ILEs),4 we provide clinicians and their pediatric patients with a variety of PN choices. From a mixed-oil blend to an omega-3-rich emulsion to a 100% soybean oil-based formulation, Fresenius Kabi offers more options for unique pediatric nutrition needs.
Explore the 4-oil difference
SMOFlipid is our proprietary ILE that helped us bring the benefits of alternative lipid emulsions to market. Designed to provide a source of calories and essential fatty acids (EFAs), SMOFlipid nourishes pediatric patients with a one-of-a-kind blend of 4 oil sources5:
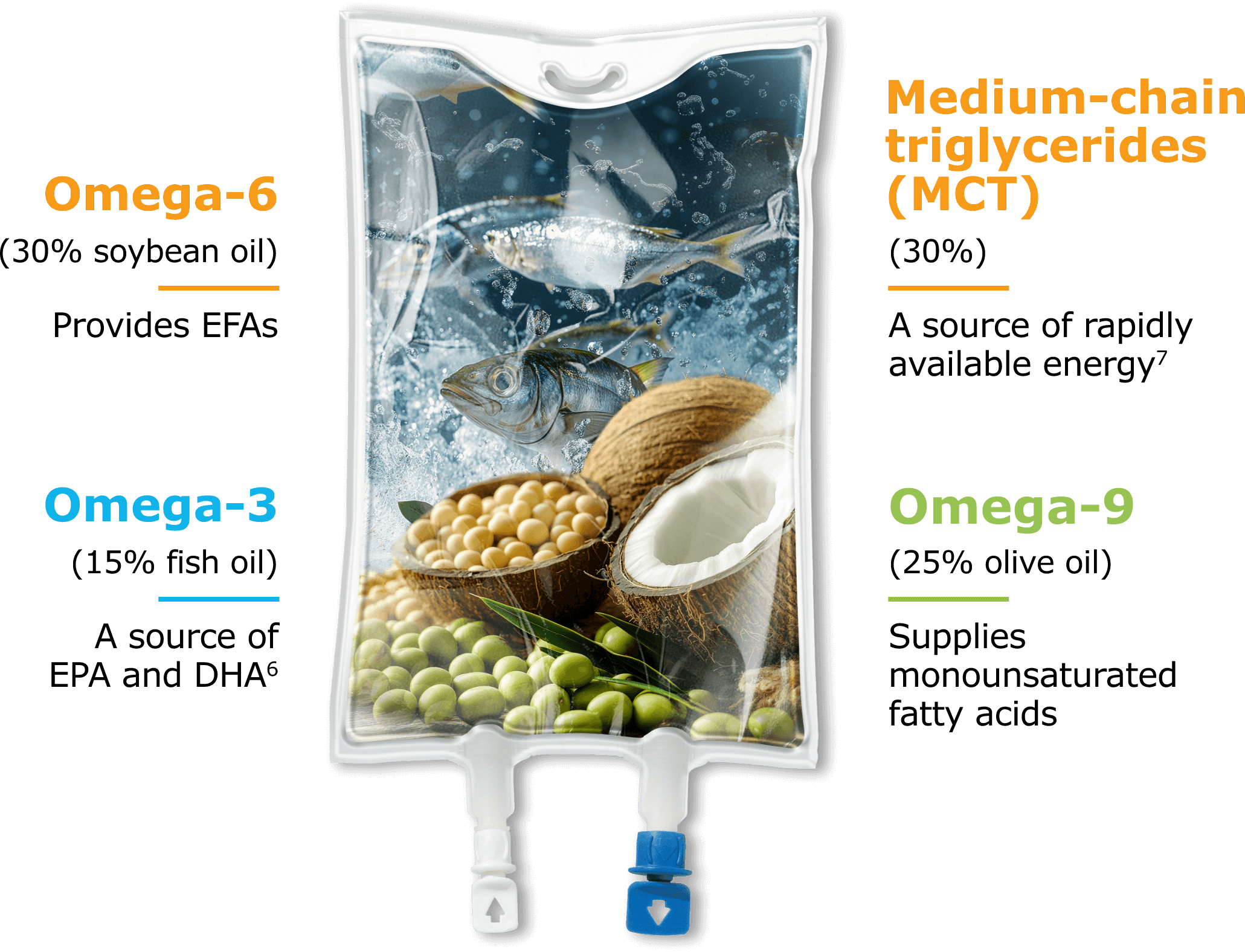
PN-associated cholestasis (PNAC) developed less frequently in pediatric patients fed a 4-oil ILE versus a 100% soybean oil (SO) ILE.5
In a randomized clinical trial among neonates and infants expected to be treated with PN for at least 28 days, PNAC, a precursor to PN-associated liver disease (PNALD), developed less frequently in SMOFlipid-treated patients than in 100% SO lipid emulsion-treated patients.5
Pediatric Study 1 also compared the incidence of PNAC (DBIL >2 mg/dL with a second confirmed DBIL >2mg/dL at least 7 days later) in both groups5:
- PNAC mostly occurred in patients who received treatment for more than 28 days
- 2.4% (2/83) of SMOFlipid-treated patients developed PNAC
- 11.5% (9/78) of SO lipid emulsion-treated patients developed PNAC
One company-sponsored trial showed equivalent total bilirubin and direct bilirubin levels comparing SMOFlipid with a soybean-based ILE.
*Data on file 11/1/24.
Nurture with omega-3-rich PN
Premature infants with PNAC may require specific nutrients. Rich in omega-3 fatty acids, Omegaven is the first and only 100% fish oil lipid emulsion in the US for pediatric patients with PNAC, and it has been shown to help achieve age-appropriate growth.8
Fresenius Kabi is committed to bringing more to pediatric PN. Thank you for partnering with us to nourish tiny patients who require clinical nutrition support.
INDICATIONS AND USAGE
Intralipid® 20% (lipid injectable emulsion) for intravenous use, Intralipid 30% (lipid injectable emulsion) for intravenous use Pharmacy Bulk Package, SMOFlipid 20%, (lipid injectable emulsion), for intravenous use
Intralipid and SMOFlipid are indicated as a source of calories and essential fatty acids for adult and pediatric patients requiring parenteral nutrition (PN) and as a source of essential fatty acids for prevention of essential fatty acid deficiency (EFAD).
Pharmacy Bulk Packages are for admixing only and are not intended for direct intravenous infusion.
Omegaven (fish oil triglycerides) injectable emulsion, for intravenous use
Omegaven is indicated as a source of calories and fatty acids in pediatric patients with parenteral nutrition-associated cholestasis (PNAC).
Limitations of Use: Omegaven is not indicated for the prevention of PNAC. It has not been demonstrated that Omegaven prevents PNAC in PN-dependent patients. It has not been demonstrated that the clinical outcomes observed in patients treated with Omegaven are a result of the omega-6:omega-3 fatty acid ratio of the product.
IMPORTANT SAFETY INFORMATION
Protect the PN admixture from light. Use a non-DEHP infusion set and 1.2 micron-inline filter during administration. Prior to administration, correct severe fluid and electrolyte disorders. Recommended dosage depends on age, energy expenditure, clinical status, body weight, ability to metabolize and eliminate lipids and additional energy given to the patient. The recommended SMOFlipid and Intralipid dose for adults and pediatrics is shown in Table 1 and recommended Omegaven dosing is shown in Table 2. For information on age-appropriate infusion rates and maximum infusion rates, see the full prescribing information. For Omegaven, initiate dosing in PN-dependent pediatric patients as soon as direct or conjugated bilirubin levels are 2 mg/dL or greater. Administer Omegaven until direct or conjugated bilirubin levels are less than 2 mg/dL or until the patient no longer requires PN.
Contraindications include the following: known sensitivity to the active or inactive ingredients; severe disorders of lipid metabolism characterized by hypertriglyceridemia (serum triglycerides >1000 mg/dL); severe hemorrhagic disorders (Omegaven).
Table 1: Recommended Dosage Intralipid 20% and SMOFlipid 20%
| Age | Nutritional Requirements | |
|---|---|---|
| Initial Recommended Dosage | Maximum Dosage | |
| Birth to 2 years of age (including preterm and term neonates) |
SMOFlipid: 0.5 to 1 g/kg/day Intralipid: 0.5 g/kg/day |
3 g/kg/day |
| Pediatric patients 2 to <12 years of age | 1 to 2 g/kg/day | SMOFlipid: 3 g/kg/day Intralipid: 2.5 g/kg/day |
| Pediatric patients 12 to 17 years of age | 1 g/kg/day | SMOFlipid: 2.5 g/kg/day Intralipid: 2 g/kg/day |
| Adults | SMOFlipid: 1 to 2 g/kg/day Intralipid: 1 g/kg/day (stable); ≤ g/kg/day (critically ill) |
2.5 g/kg/day |
Table 2: Recommended Omegaven and Infusion Rate
| Nutritional Requirements | Direct Infusion Rate | |
|---|---|---|
| Recommended Initial Dosage and Maximum Dosage |
Initial | Maximum |
| 1 g/kg/day; this is also the maximum daily dose |
0.2 mL/kg/hour for the first 15 to 30 minutes; gradually increase to the required rate after 30 minutes |
1.5 mL/kg/hour |
Clinical Decompensation with Rapid Infusion of Intravenous Lipid Emulsion in Neonates and Infants: Acute respiratory distress, metabolic acidosis, and death after rapid infusion of intravenous lipid emulsions have been reported. Strictly adhere to the hourly infusion rate. Carefully monitor the infant’s ability to eliminate the infused lipids from the circulation (e.g., measure serum triglycerides and/or plasma free fatty acid levels). If signs of poor clearance of lipids from the circulation occur, stop the infusion and initiate a medical evaluation. When Intralipid 30% is diluted to 20%, strictly adhere to the recommended total daily dosage; the hourly infusion rate should not exceed 0.125 g/kg/hour for neonates and infants.
Parenteral Nutrition-Associated Liver Disease (PNALD): Increased risk in patients who receive PN for extended periods of time, especially preterm neonates. Monitor liver function tests; if abnormalities occur consider discontinuation or dosage reduction (Intralipid and SMOFlipid).
Hypersensitivity Reactions: Monitor for signs or symptoms. Discontinue infusion if reactions occur.
Risk of Infections, Fat Overload Syndrome, Refeeding Syndrome, and Hypertriglyceridemia and Essential Fatty Acid Deficiency: Monitor for signs and symptoms; monitor laboratory parameters.
Aluminum Toxicity: Increased risk in patients with renal impairment, including preterm neonates
Monitoring and Laboratory Tests: Routine laboratory monitoring is recommended, including monitoring for essential fatty acid deficiency.
Intralipid and SMOFlipid: Most common adverse drug reactions (≥5%) from clinical trials in adults were nausea, vomiting (SMOFlipid and Intralipid), pyrexia (Intralipid) and hyperglycemia (SMOFlipid). Most common adverse drug reactions (≥5%) from clinical trials in pediatric patients were anemia, vomiting, increased gamma-glutamyltransferase, cholestasis (Intralipid), and nosocomial infection (SMOFlipid).
Intralipid and SMOFlipid: Vitamin K Antagonists (e.g., warfarin): Anticoagulant activity may be counteracted; increase monitoring of coagulation parameters.
Omegaven: The most common adverse drug reactions (>15%) are: vomiting, agitation, bradycardia, apnea and viral infection.
Omegaven: Antiplatelet Agents and Anticoagulants: Prolonged bleeding time has been reported in patients taking antiplatelet agents or anticoagulants and oral omega-3 fatty acids. Periodically monitor bleeding time in patients receiving Omegaven and concomitant antiplatelet agents or anticoagulants.
To report SUSPECTED ADVERSE REACTIONS, contact Fresenius Kabi USA, LLC at 1-800-551-7176, option 5, or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
This Important Safety Information does not include all the information needed to use SMOFlipid, Intralipid and Omegaven safely and effectively. Please see full prescribing information, for SMOFlipid, Intralipid and Omegaven at www.FreseniusKabiNutrition.com/SMOFlipidPI, www.FreseniusKabiNutrition.com/Intralipid20PI, www.FreseniusKabiNutrition.com/Intralipid30PI, and www.FreseniusKabiNutrition.com/OmegavenPI.

