Category: Intellectual Nourishment

Nourishment without disruption: tips for maintaining continuity of care
Parenteral nutrition (PN) is a complex therapy that may be administered to patients at any age in a variety of clinical settings, including hospitals, long-term care (LTC) facilities, and at home.1 Because it is classified as a high-alert medication by the Institute for Safe Medication Practices,2,3 PN requires an interdisciplinary approach to help mitigate potential risks and complications associated with its use.1 This is especially important when patients who are receiving PN are transferred from one clinical setting to another.
Established coordination and clear communication are key to facilitating successful transitions from4:
- One medical unit to another within a hospital
- A hospital to a skilled nursing facility, LTC facility, or home
- A skilled nursing facility, LTC facility, or home to a hospital
Care transitions come with challenges
Transitions of care pose several challenges not only for healthcare providers and organizations but for patients and caregivers.4 It’s been shown that hospital discharge is associated with risks that cause medical errors and adverse events that can lead to readmission and/or complications.4,5 These include failure of communication, insufficient patient education, lack of timely follow-up, and inadequate home services.5 While these things can negatively impact a patient’s journey on PN, there are steps that can be taken to achieve a transition of care that is safe and successful.
“When a high-alert medication such as PN is involved, risk-avoidance strategies require standardized processes with excellent communication between all members of the team, including healthcare providers from the sending and receiving organization/setting who have a high degree of competence in their respective roles as well as the patient/caregiver.”4
From: Adams SC, et al. Nutr Clin Pract. 2022;37(3):493-508.
Considerations for navigating transitions of care for PN patients
When it comes to transferring patients, safety is the top priority. Unfortunately, medication errors affect almost every patient whose care is transitioned to another clinical setting.6 To reduce the risk of these errors, a standardized process should be considered to ensure all aspects of the transition are addressed.4
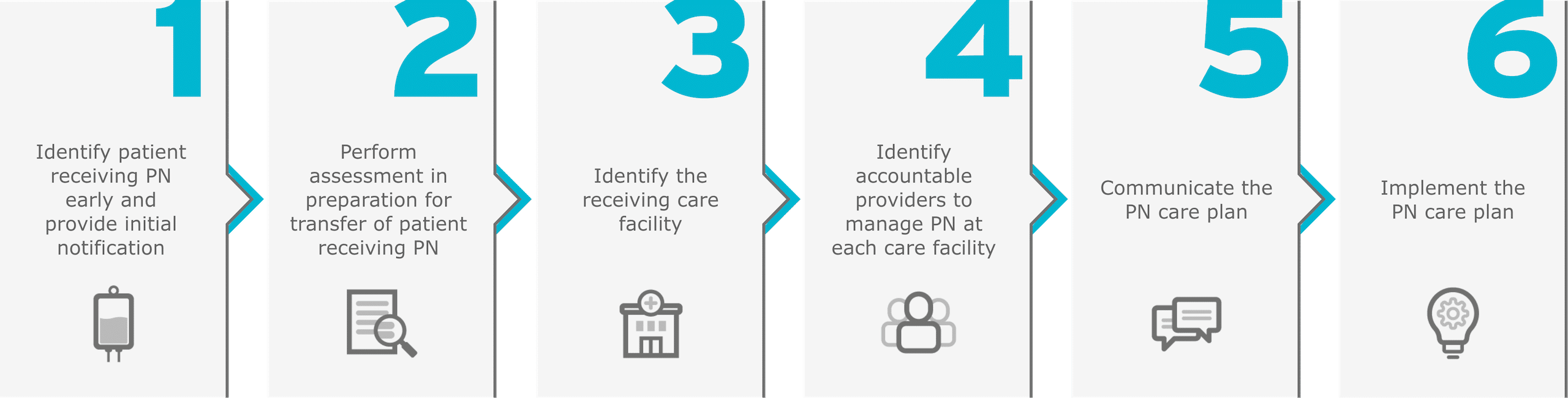
Phases in the transition process4
Adapted from: Adams SC, et al. Nutr Clin Pract. 2022;37(3):493-508.
This sample checklist is just one step toward creating a systematic approach to transitioning PN patients from one clinical setting to another4:
-
- Evaluate the necessity of PN based on medical condition, nutritional requirements, and anticipated duration
-
- Assign responsibilities to team members involved in the transition process, including physicians, nurses, pharmacists, dietitians, and home healthcare providers
-
- Conduct a thorough review of the patient’s current medications and allergies, ensuring compatibility and safety of PN
-
- Schedule follow-up appointments with healthcare providers, including physicians, dietitians, and pharmacists, to monitor the patient’s progress and adjust PN therapy as needed
-
- Provide comprehensive education to the patient and caregivers regarding PN administration, equipment uses, and signs of complications
- Address nutritional needs, dietary restrictions, and lifestyle modifications
-
- Verify the availability of PN products and supplies needed for home administration
- Make adjustments to the PN order as necessary, considering formulary differences and addressing any product shortages
- Communicate changes with the care team and ensure proper documentation
-
- Verify and review the PN order to ensure it is appropriate, accurate, complete, stable, and compatible
- Ensure adherence to aseptic techniques and proper handling of PN components
-
- Develop protocols for preventing central line-associated bloodstream infections (CLABSIs), including catheter care, dressing changes, and sterile techniques
- Educate caregivers on infection prevention measures and early signs of infection
-
- Develop contingency plans for potential complications or readmissions related to PN therapy
- Communicate these plans to the patient, caregivers, and healthcare providers involved in the transition process
-
- Monitor the clinical outcomes and financial implications of the transition process
- Identify and address any challenges or barriers to successful transition, considering both clinical and financial factors
Careful coordination, recognizing challenges that may present during transitions of care, and following the necessary policies and procedures can all help reduce the risk of errors that may lead to patient harm.4 Every team member and every stage of the process is critical to ensuring a smooth and successful care transition for healthcare providers, their patients receiving PN, and caregivers.4
Fresenius Kabi is proud to partner with clinicians who ensure patients receive the clinical nutrition products they need, no matter the setting.
Sources: 1. Boullata JI. Overview of the parenteral nutrition use process. JPEN J Parenter Enteral Nutr. 2012;36(2 Suppl):10S-13S. 2. ISMP List of High-Alert Medications in Acute Care Settings. Institute for Safe Medication Practices (ISMP). 2024. 3. High-Alert Medications in Long-Term Care (LTC) Settings. Institute for Safe Medication Practices (ISMP). 2021. 4. Adams SC, Gura KM, Seres DS, et al. Safe care transitions for patients receiving parenteral nutrition. Nutr Clin Pract. 2022;37(3):493-508. 5. Greenwald JL, Denham CR, Jack BW. The Hospital Discharge: A Review of a High Risk Care Transition With Highlights of a Reengineered Discharge Process. J Patient Saf. 2007;3(2):97-105. 6. World Health Organization. Medication Safety in Transitions of Care Technical Report. 2019. Accessed March 6, 2024. https://iris.who.int/bitstream/handle/10665/325453/WHO-UHC-SDS-2019.9-eng.pdf?sequence=1

Importance of compatibility and stability in parenteral nutrition (PN)
Many people in the US require PN to help them meet their nutritional needs. In fact, more than 33,000 hospitalized patients receive PN while an estimated 25,000 patients require PN at home.1,2 Some patients may even receive PN in long-term care (LTC) facilities.
While PN is considered a valued clinical intervention for those who need it, it is complex, and clinicians must follow safety-focused policies, procedures, and practices—no matter the clinical setting.3
The Institute for Safe Medication Practices classifies PN as a high-alert medication in acute care and LTC settings, meaning that significant patient harm can occur if PN is used in error.4,5 Errors can occur at any step of the PN process, including review and verification of the order by a pharmacist.6 This step is critical for evaluating the compatibility and stability of the PN admixture before it goes out the door.3,6 Understanding compatibility and stability are important considerations for ensuring a patient receives PN that is safe and effective.
“The critical step of PN order verification and review by the pharmacist requires a contextual assessment of the compatibility and stability implications of the ordered PN prescription.”3
There are various components used when compounding PN. These may include lipid emulsions, amino acids, and electrolytes, and each component has unique physicochemical properties that can affect the compatibility and stability of the final admixture.3
![]()
Compatibility simply means that two or more components may be combined without resulting in unfavorable physical or chemical reactions over time.3 However, if the components are incompatible, adverse reactions may occur or the therapeutic efficacy of one or more of the components may be decreased.3
![]()
Stability means an ingredient or a dosage form is able to maintain its chemical and physical properties throughout its shelf life and storage.3 Temperature, light, oxygen, solvents, reactants, and pH can all cause instability, resulting in decomposition or degradation of the ingredient or dosage form that cannot be reversed.3
When it comes to PN, a pharmacist must be knowledgeable in pharmaceutics and understand how to apply known data on the physical and chemical properties of each component to best determine compatibility and stability.3 Altering just one component can impact the final admixture, no matter if the PN is customized for a patient’s specific nutritional needs or if a standardized commercially available PN product is used.3
“When you talk about stability, you have to look at the macronutrients that you’re compounding the PN with, number one. And number two, you have to look at the final concentration of each macronutrient.”
Reid Nishikawa, PharmD, BCNSP, FASPEN, FCSHP
As the US market leader in lipid injectable emulsions (ILEs),7 we recognize that compatibility—and particularly, stability—can pose a challenge when considering the use of ILEs. That’s why we worked tirelessly on obtaining the necessary research to generate compatibility and stability data for PN admixtures involving two of our alternative ILEs. To view our Compatibility Tool and Stability Data Reference Guides, please visit: www.freseniuskabinutrition.com/resources/#products
References: 1. John J, Seifi A. Total parenteral nutrition usage trends in the United States. J Crit Care. 2017;40:312-313. 2. Mundi MS, Pattinson A, McMahon MT, Davidson J, Hurt RT. Prevalence of Home Parenteral and Enteral Nutrition in the United States. Nutr Clin Pract. 2017;32(6):799-805. 3. Boullata JI, Mirtallo JM, Sacks GS, et al. Parenteral nutrition compatibility and stability: A comprehensive review. JPEN J Parenter Enteral Nutr. 2022;46(2):273-299. 4. ISMP List of High-Alert Medications in Acute Care Settings. ISMP website. 2018. Accessed January 9, 2024. https://www.ismp.org/sites/default/files/attachments/2018-08/highAlert2018-Acute-Final.pdf 5. High-Alert Medications in Long-Term Care (LTC) Settings. ISMP website. 2021. Accessed January 9, 2024. https://www.ismp.org/system/files/resources/2021-05/HighAlertMedications_LTC-2021.pdf 6. Mirtallo JM, Ayers P. Parenteral Nutrition Safety. Pharmacy Practice News website. September 7, 2021. Accessed January 9, 2024. https://www.pharmacypracticenews.com/Review-Articles/Article/09-21/Parenteral-Nutrition-Safety/64518 7. Data on File; 1/1/24; calculation includes: all ILEs approved in the US.

Navigating lipid sources in parenteral nutrition (PN)
Because lipid injectable emulsions (ILEs) are an energy-dense source of calories in PN solutions, choosing a lipid source is important. In this blog post, we’ll discuss various lipid sources and compare 2 mixed-oil ILEs.
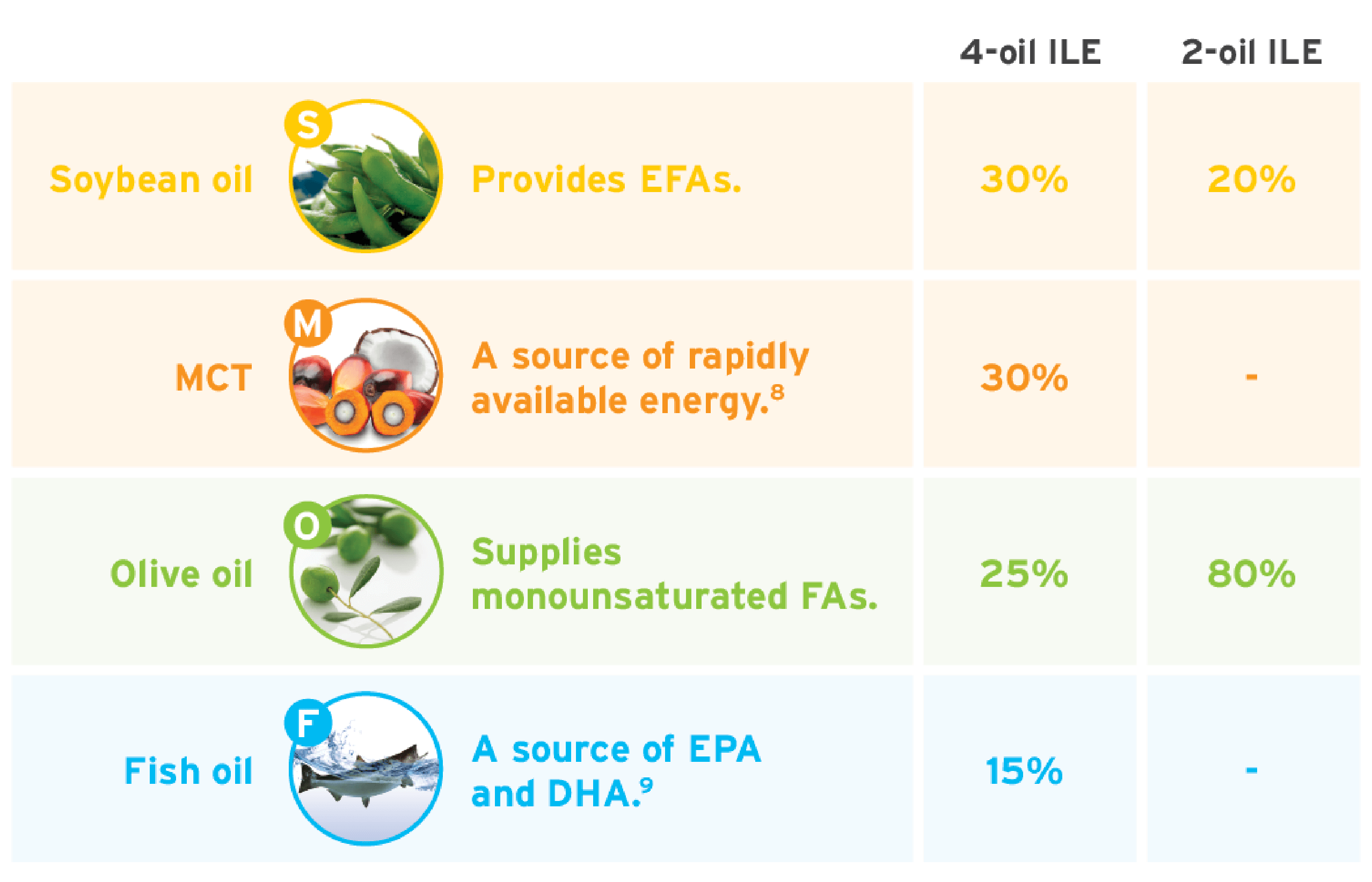
Evaluating lipid sources: A variety of lipid sources are available for PN, including medium-chain triglycerides (MCTs), olive oils, and fish oils, which, based on extensive usage in Europe, have an equivalent safety profile to soybean oil.1 These alternative ILEs are metabolized via different pathways.1
Mixed-oil ILEs: While standard soybean oil-based emulsions are commonly used, alternative mixed-oil emulsions containing omega-3s and omega-6s can be a unique blend due to their diverse fatty acid content.
Tailoring nutrition: Recognizing that each patient is unique, it is important to take into consideration the patient’s age, clinical condition, therapeutic objectives, and tolerance when considering a lipid dose.
The role of clinical expertise: The expertise of healthcare professionals is paramount in the selection and management of lipid sources in PN. Ongoing assessment and monitoring of patients is integral to ensure the appropriate choice of lipid for each patient.
An informed approach is critical to incorporating mixed-oil ILEs as a part of the clinical management of patients. Alternative lipid sources containing fish oil are the 4th generation of ILEs. Specifically, a 4-oil ILE was the most recent innovation to the market, receiving approval for adults in 2016 and pediatrics in 2022.
Use this table as a quick reference on the compositional differences between 2 mixed-oil ILEs on the market today.
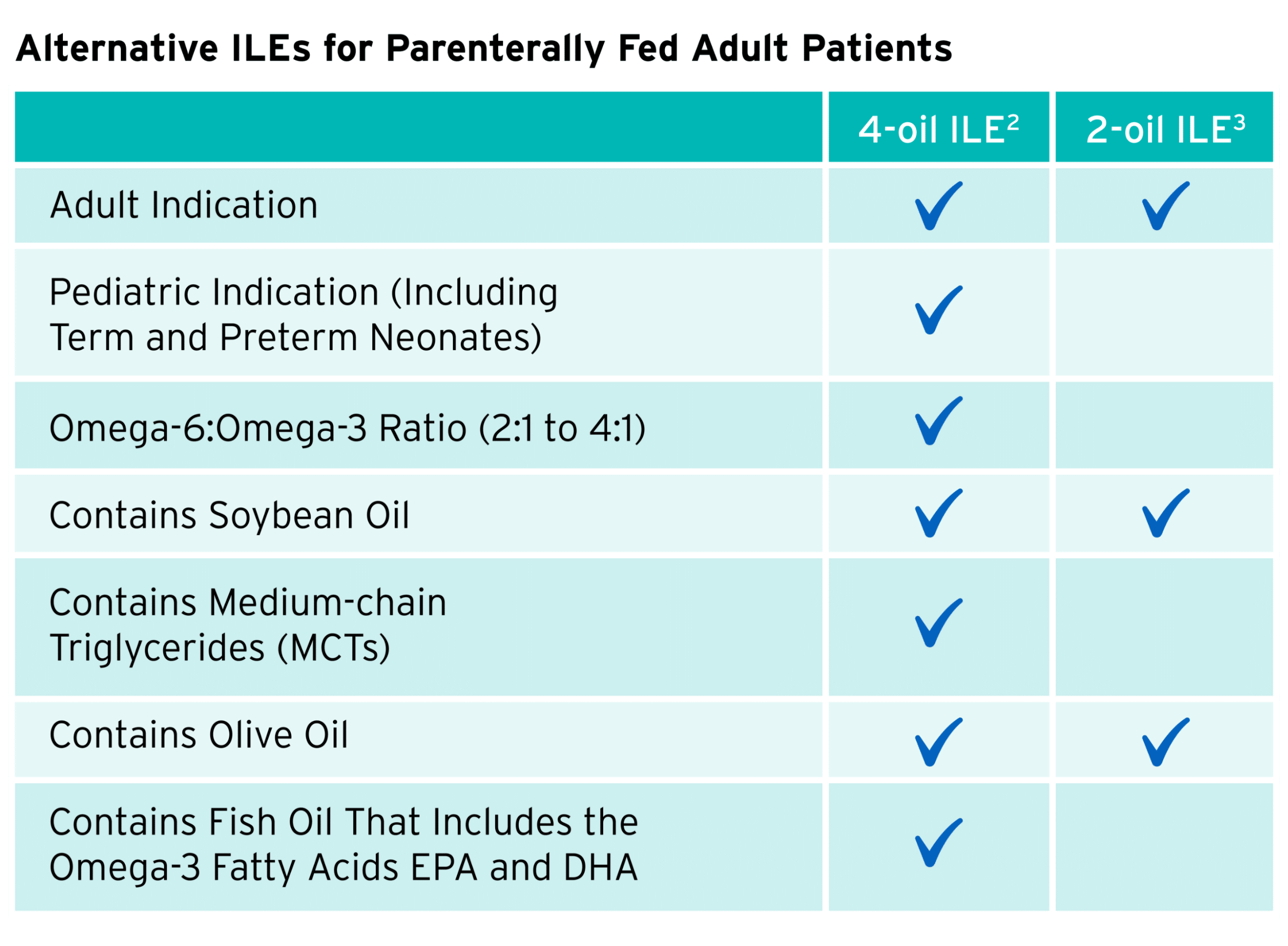
Expert recommendations suggest an omega-6:omega-3 ratio of 2:1 to 4:1 in ILEs.4-7
At Fresenius Kabi, our dedication to caring also means we’re dedicated to keeping healthcare professionals informed. Staying current with the latest research and news in the field of PN can help you educate patients about the treatments they receive, including the importance of PN and the specific products being used.
Looking for more information?
SMOFlipid® (lipid injectable emulsion, USP), for intravenous use
IMPORTANT SAFETY INFORMATION
What is SMOFlipid?
- Indicated in adult and pediatric patients as a source of calories and essential fatty acids for parenteral nutrition (PN) when oral or enteral nutrition is not possible, insufficient, or contraindicated.
- The hourly infusion rate in pediatrics should not exceed 0.75 mL/kg/hour and 0.5 mL/kg/hour in adults.
SMOFlipid should not be received by patients who have:
- A known allergy to fish, egg, soybean, or peanut, or to any of the active or inactive ingredients in SMOFlipid.
- Abnormally high levels of lipid (triglycerides) in the blood.
SMOFlipid may cause serious side effects including:
- Serious Adverse Reactions with Rapid Infusion of Intravenous Lipid Emulsion in Neonates and Infants: Strictly follow the recommended total daily dosage and do not exceed the maximum infusion rate. If poor clearance of fats occurs, the infusion should be stopped, and a medical evaluation started.
- Risk of Parenteral Nutrition-Associated Liver Disease: Parenteral nutrition-associated liver disease (PNALD) may progress to liver inflammation and damage caused by a buildup of fat in the liver with scarring and cirrhosis.
- Allergic Reactions: Contact your healthcare provider immediately if you are experiencing an allergic reaction.
- Fat Overload Syndrome, Refeeding Syndrome, Elevated Triglycerides (Hypertriglyceridemia): Your healthcare provider will monitor you for signs and symptoms of early infection and blood levels.
Monitoring/Laboratory Tests: The content of vitamin K may interfere with blood clotting activity of medications.
The most common side effects (>1%) in adult patients include nausea, vomiting, and high levels of glucose in the blood and in pediatric patients include low levels of red blood cells, vomiting, increased levels of liver enzymes (i.e., gamma-glutamyltransferase) and hospital-acquired infections.
These are not all the possible side effects associated with SMOFlipid. Call your healthcare provider for medical advice regarding SMOFlipid side effects. You are encouraged to report negative side effects of SMOFlipid. Contact Fresenius Kabi USA, LLC at: 1-800-551-7176 or FDA at: 1-800-FDA-1088 or www.fda.gov/medwatch. The FDA-approved product labeling can be found at https://www.freseniuskabinutrition.com/SMOFlipidPI.
References: 1. Vanek VW, Seidner DL, Allen P, et al. A.S.P.E.N. position paper: Clinical role for alternative intravenous fat emulsions. Nutr Clin Pract. 2012;27(2):150-192. 2. SMOFlipid Prescribing Information, Fresenius Kabi USA, LLC. 2023. 3. Clinolipid Prescribing Information, Baxter Healthcare Corporation. 2023. 4. Grimble RH. Fatty acid profile of modern lipid emulsions: scientific consideration for creating the ideal composition. Clin Nutr Suppl. 2005;1(3):9-15. 5. Waitzberg DL, Torrinhas RS, Jacintho TM. New parenteral lipid emulsions for clinical use. JPEN J Parenter Enteral Nutr. 2006;30(4):351-367. 6. Mayer K, Schaefer MB, Seeger W. Fish oil in the critically ill: from experimental to clinical data. Curr Opin Clin Nutr Metab Care. 2006;9(2):140-148. 7. Grimm H, Mertes N, Goeters C, et al. Improved fatty acid and leukotriene pattern with a novel lipid emulsion in surgical patients. Eur J Nutr. 2006;45(1):55-60. 8. Deckelbaum RJ, Hamilton JA, Moser A, et al. Medium-chain versus long-chain triacylglycerol emulsion hydrolysis by lipoprotein lipase and hepatic lipase: implications for the mechanisms of lipase action. Biochemistry. 1990;29(5):1136-1142. 9. Kalish BT, Fallon EM, Puder M. A tutorial on fatty acid biology. JPEN J Parenter Enteral Nutr. 2012;36(4):380-388.
Fresenius Kabi: at the forefront of parenteral nutrition (PN)
Fresenius Kabi is a global healthcare company that specializes in lifesaving medicines and technologies, including clinical nutrition and infusion therapy. Our PN products provide nourishment that helps to care for critically and chronically ill patients all around the world.
Built on years of innovation
Our history begins more than a century ago when Dr. Eduard Fresenius founded the pharmaceutical company Dr. E. Fresenius. There, he offered several pharmaceutical specialty medications, including injectable solutions. Since then, Fresenius Kabi has supported the research needed to drive advancements in clinical nutrition and deliver options to healthcare providers and their patients on PN. We have built on decades of innovation, and our tireless dedication has led to many “firsts” in the field of PN.
A history of “firsts”

In 2014, Fresenius Kabi launched the first and only three-chamber bags for adult PN in the US, Kabiven® (Amino Acids, Electrolytes, Dextrose, and Lipid Injectable Emulsion), for intravenous use and Perikabiven® (Amino Acids, Electrolytes, Dextrose, and Lipid Injectable Emulsion), for intravenous use.1,2

In 2016, Fresenius Kabi pioneered the use of fish oil and omega-3s in PN products. During that same year, we brought the benefits of alternative lipid emulsions to market.

In 2018, the FDA approved Omegaven® (fish oil triglycerides) injectable emulsion, the first and only 100% fish oil lipid injectable emulsion (ILE) developed by Fresenius Kabi for pediatric patients with PN-associated cholestasis (PNAC) in the US.3

In 2022, the first four-oil ILE, SMOFlipid® (Lipid Injectable Emulsion, USP 20%), was approved for pediatric patients, including term and preterm neonates.4

In 2023, Fresenius Kabi welcomed Intralipid® (lipid injectable emulsion, USP 20%), a soybean oil-based ILE, back home.5

Fresenius Kabi continues to drive innovation in the world of clinical nutrition.
Caring for life
Fresenius Kabi’s purpose is “caring for life,” and we’re committed to putting lifesaving medicines and technologies in the hands of people who care for patients and finding answers to the challenges they face. We offer an array of PN resources, including detailed product information, case studies, educational videos, and helpful guides. Check them out: www.FreseniusKabiNutrition.com/resources/
Kabiven (Amino Acids, Electrolytes, Dextrose, and Lipid Injectable Emulsion), for intravenous use and Perikabiven (Amino Acids, Electrolytes, Dextrose, and Lipid Injectable Emulsion), for intravenous use
IMPORTANT SAFETY INFORMATION
What is Kabiven and Perikabiven?
- Indicated in adult patients as a source of calories, protein, electrolytes and essential fatty acids for parenteral nutrition when oral or enteral nutrition is not possible, insufficient, or contraindicated. Kabiven and Perikabiven may be used to prevent essential fatty acid deficiency or treat negative nitrogen balance in adults.
- Do not exceed the recommended maximum infusion rate of 2.6 mL/kg/hour for Kabiven and 3.7 mL/kg/hour for Perikabiven.
Limitations of Use
Neither Kabiven nor Perikabiven is recommended in pediatric patients less than 2 years old because the fixed amount of the formulations do not meet nutritional needs in this age group.
Do not use Kabiven or Perikabiven in patients who have:
- Simultaneous treatment with ceftriaxone in neonates (28 days of age or younger)
- Known allergy to egg, soybean, peanut or any of the active or inactive ingredients
- Abnormally high levels of lipid (triglycerides) in the blood (with serum triglyceride concentration >1,000 g/dL)
- Inborn errors of amino acid metabolism (a genetic defect in protein metabolism)
- Cardiopulmonary instability (inability for the heart and lungs to function right)
- Hemophagocytic syndrome (a disorder of the immune system)
Kabiven and Perikabiven may cause serious side effects including:
- Serious Adverse Reactions with Rapid Infusion of Intravenous Lipid Emulsion in Neonates and Infants: Strictly follow the recommended total daily dosage and do not exceed the maximum infusion rate. If poor clearance of fats occurs, the infusion should be stopped, and a medical evaluation started.
- Risk of Parenteral Nutrition-Associated Liver Disease: Increased risk in patients who receive parenteral nutrition for greater than 2 weeks. Your healthcare provider will monitor liver tests.
- Pulmonary Embolism (a blockage in a blood vessel in the lung) and Respiratory Distress (increased breathing rate, bluish skin color changes, wheezing) due to Pulmonary Vascular Precipitates (solid substance in the blood vessel of the lungs): If signs of lung issues occur, stop the infusion and start a medical evaluation.
- Allergic Reactions: Contact your healthcare provider immediately if you are experiencing an allergic reaction
- Precipitation (solid substance in the blood vessel) with Ceftriaxone: Do not administer ceftriaxone simultaneously with Kabiven or Perikabiven via a Y-site.
- Infection, fat overload, hyperglycemia (high blood sugar) and refeeding syndrome: Your healthcare provider will monitor you for signs and symptoms of early infection and blood levels
The most common adverse reactions for Kabiven (≥3%) are nausea, fever, high blood pressure, vomiting, decreased blood hemoglobin, decreased blood total protein, low blood potassium, and increased gamma glutamyltransferase (a liver enzyme). The most common adverse reactions for Perikabiven (≥3%) are high blood sugar, low blood potassium, fever and increased blood lipids.
To report SUSPECTED ADVERSE REACTIONS, contact Fresenius Kabi USA, LLC at 1-800-551-7176, option 5, or FDA at 1-800-FDA-1088 orwww.fda.gov/medwatch.
Tell your doctor if you are taking coumarin and coumarin derivatives, including warfarin: the drug activity may be lessened and your healthcare provider will monitor your blood.
These are not all the possible side effects associated with Kabiven and Perikabiven. Call your healthcare provider for medical advice regarding Kabiven and Perikabiven side effects. You are encouraged to report negative side effects of Kabiven and Perikabiven. Contact Fresenius Kabi USA, LLC at: 1-800-551-7176 or FDA at: 1-800-FDA-1088 or www.fda.gov/medwatch. The FDA-approved product labeling can be found at www.freseniuskabinutrition.com/KabivenPI and www.freseniuskabinutrition.com/PeriKabivenPI.
OMEGAVEN (fish oil triglycerides) injectable emulsion, for intravenous use
IMPORTANT SAFETY INFORMATION
These highlights do not include all the information needed to use OMEGAVEN safely and effectively. To learn more about OMEGAVEN for your child, talk to your child’s healthcare provider. OMEGAVEN is available by prescription only. The FDA-approved product labeling can be found at www.freseniuskabinutrition.com/OmegavenPI.
What is OMEGAVEN?
- A fish oil-based intravenous lipid emulsion that is a source of calories and fatty acids in pediatric patients with parenteral nutrition-associated cholestasis (PNAC).
- Does not prevent PNAC.
- It has not been demonstrated that the clinical outcomes seen in pediatric patients are a result of the omega-6:omega-3 fatty acid ratio of the product.
- The hourly infusion rate should not exceed 1.5 mL/kg/hour
OMEGAVEN should not be received by patients who have:
- a known allergy to fish or egg protein or to any of the ingredients in OMEGAVEN.
- a severe bleeding disorder.
- abnormally high levels of lipid (triglycerides) in the blood.
What important safety information should I know about OMEGAVEN?
- Serious Adverse Reactions with Rapid Infusion of Intravenous Lipid Emulsion in Neonates and Infants: Strictly follow the recommended total daily dosage and do not exceed the maximum infusion rate. If poor clearance of fats occurs, the infusion should be stopped, and a medical evaluation started.
- Allergic Reactions: Contact your healthcare provider immediately if you are experiencing an allergic reaction.
- Fat Overload Syndrome, Refeeding Syndrome, Elevated Triglycerides (Hypertriglyceridemia): Your healthcare provider will monitor you for signs and symptoms of early infection and blood levels.
The most common side effects, (>15%) include: vomiting, agitation, slower than normal heartbeat, interruption of breathing, and viral infection.
These are not all the possible side effects associated with OMEGAVEN. Call your healthcare provider for medical advice regarding OMEGAVEN side effects. You are encouraged to report negative side effects of OMEGAVEN. Contact Fresenius Kabi USA, LLC at: 1-800-551-7176 or FDA at: 1-800-FDA-1088 or www.fda.gov/medwatch. The FDA-approved product labeling can be found at www.freseniuskabinutrition.com/OmegavenPI.
SMOFlipid® (lipid injectable emulsion, USP), for intravenous use
IMPORTANT SAFETY INFORMATION
What is SMOFlipid?
- Indicated in adult and pediatric patients as a source of calories and essential fatty acids for parenteral nutrition (PN) when oral or enteral nutrition is not possible, insufficient, or contraindicated.
- The hourly infusion rate in pediatrics should not exceed 0.75 mL/kg/hour and 0.5 mL/kg/hour in adults.
SMOFlipid should not be received by patients who have:
- A known allergy to fish, egg, soybean, or peanut, or to any of the active or inactive ingredients in SMOFlipid.
- Abnormally high levels of lipid (triglycerides) in the blood.
SMOFlipid may cause serious side effects including:
- Serious Adverse Reactions with Rapid Infusion of Intravenous Lipid Emulsion in Neonates and Infants: Strictly follow the recommended total daily dosage and do not exceed the maximum infusion rate. If poor clearance of fats occurs, the infusion should be stopped, and a medical evaluation started.
- Risk of Parenteral Nutrition-Associated Liver Disease: Parenteral nutrition-associated liver disease (PNALD) may progress to liver inflammation and damage caused by a buildup of fat in the liver with scarring and cirrhosis.
- Allergic Reactions: Contact your healthcare provider immediately if you are experiencing an allergic reaction.
- Fat Overload Syndrome, Refeeding Syndrome, Elevated Triglycerides (Hypertriglyceridemia): Your healthcare provider will monitor you for signs and symptoms of early infection and blood levels.
Monitoring/Laboratory Tests: The content of vitamin K may interfere with blood clotting activity of medications.
The most common side effects (>1%) in adult patients include nausea, vomiting, and high levels of glucose in the blood and in pediatric patients include low levels of red blood cells, vomiting, increased levels of liver enzymes (i.e., gamma-glutamyltransferase) and hospital-acquired infections.
These are not all the possible side effects associated with SMOFlipid. Call your healthcare provider for medical advice regarding SMOFlipid side effects. You are encouraged to report negative side effects of SMOFlipid. Contact Fresenius Kabi USA, LLC at: 1-800-551-7176 or FDA at: 1-800-FDA-1088 or www.fda.gov/medwatch. The FDA-approved product labeling can be found at www.freseniuskabinutrition.com/SMOFlipidPI.
Intralipid (lipid injectable emulsion) for intravenous use
IMPORTANT SAFETY INFORMATION
What is Intralipid?
- Indicated as a source of calories and essential fatty acids for adult and pediatric patients requiring parenteral nutrition (PN) and as a source of essential fatty acids for prevention of essential fatty acid deficiency (EFAD).
Intralipid should not be received by patients who have:
- A known allergy to egg, soybean, or peanut, or any of the active ingredients or excipients in Intralipid.
- Abnormally high levels of lipid (triglycerides) in the blood.
Intralipid may cause serious side effects including:
- Serious Adverse Reactions with Rapid Infusion of Intravenous Lipid Emulsion in Neonates and Infants: Strictly adhere to the recommended total daily dosage and do not exceed the maximum infusion rate. If poor clearance of fats occurs, the infusion should be stopped, and a medical evaluation started.
- Risk of Parenteral Nutrition-Associated Liver Disease: Parenteral nutrition-associated liver disease (PNALD) may progress to liver inflammation and damage caused by a buildup of fat in the liver with scarring and cirrhosis.
- Allergic Reactions: Contact your healthcare provider immediately if you are experiencing an allergic reaction.
- Fat Overload Syndrome, Refeeding Syndrome, Elevated Triglycerides: Your healthcare provider will monitor you for signs and symptoms of early infection and blood levels
Monitoring/Laboratory Tests: The content of vitamin K may interfere with blood clotting activity of medications.
The most common side effects (≥5%) in adult patients include nausea, vomiting and fever and in pediatric patients include low levels of red blood cells, vomiting, increased levels of liver enzymes (i.e., gamma-glutamyltransferase), and cholestasis (i.e., reducing or blocking the flow of bile).
These are not all the possible side effects associated with Intralipid. Call your healthcare provider for medical advice regarding Intralipid side effects. You are encouraged to report negative side effects of Intralipid. Contact Fresenius Kabi USA, LLC at: 1-800-551-7176 or FDA at: 1-800-FDA-1088 or www.fda.gov/medwatch. The FDA-approved product labeling can be found at www.FreseniusKabiNutrition.com/IntralipidPI.
References: 1. Kabiven Prescribing Information, Fresenius Kabi USA, LLC. 2023. 2. Perikabiven Prescribing Information, Fresenius Kabi USA, LLC. 2023. 3. Omegaven Prescribing Information, Fresenius Kabi USA, LLC. 2023. 4. SMOFlipid Prescribing Information, Fresenius Kabi USA, LLC. 2023. 5. Intralipid Prescribing Information, Fresenius Kabi USA, LLC. 2023.

Pediatric malnutrition and the role of parenteral nutrition (PN)
What is pediatric malnutrition?
Malnutrition is a big problem—not just in the U.S. but around the world. According to Dr. Francesco Branca, Director of the Department of Nutrition for Health and Development at the World Health Organization, “Malnutrition is a complex issue, but it is the main cause of death and disease in the world.”1 And it’s no respecter of age. Babies, children, and teenagers can suffer from the detrimental effects of malnutrition.
Some studies have reported that up to 51% of hospitalized pediatric patients suffer from malnutrition due to a disease or injury.2-4
Pediatric malnutrition is defined as “an imbalance between nutrient requirements and intake that results in cumulative deficits of energy, protein, or micronutrients that may negatively affect growth, development, and other relevant outcomes.”5 It may be caused by a disease or injury, which is usually the case in developed countries, and/or it may be caused by environmental, socioeconomic, or behavioral factors that lead to decreased nutrient intake.5 Although pediatric malnutrition is common, “its true prevalence is underreported and not well understood.”6
Babies, children, and teenagers who do not receive the nutrition they need to support adequate growth and development may experience negative consequences. Symptoms may include a thin or bloated appearance or a weakened immune system.7 Patients may also exhibit mood changes, faltering growth, and low energy levels.7,8
What impact does pediatric malnutrition have on patient outcomes?
Malnutrition can lead to adverse outcomes, including longer hospital stays and increased healthcare costs.4,9 Additionally, it can have lasting negative effects on pediatric patients, including stunting, which “often results in delayed mental development, poor school performance, and reduced intellectual capacity.”10
Nourishing pediatric patients with PN
Pediatric patients unable to receive adequate nutrition by mouth or enteral feeding may require PN to meet their nutritional goals.11 PN regimens often include lipid injectable emulsions (ILEs), which provide a noncarbohydrate source of energy and essential fatty acids.12,13
At Fresenius Kabi, we are dedicated to providing more options for patients who need PN.
References: 1. Malnutrition is a world health crisis. World Health Organization website. September 26, 2019. Accessed July 28, 2023. https://www.who.int/news/item/26-09-2019-malnutrition-is-a-world-health-crisis. 2. Hendricks KM, Duggan C, Gallagher L, et al. Malnutrition in hospitalized pediatric patients. Current prevalence. Arch Pediatr Adolesc Med. 1995;149(10):1118-1122. 3. Pawellek I, Dokoupil K, Koletzko B. Prevalence of malnutrition in paediatric hospital patients. Clin Nutr. 2008;27(1):72-76. 4. Secker DJ, Jeejeebhoy KN. Subjective Global Nutritional Assessment for children. Am J Clin Nutr. 2007;85(4):1083-1089. 5. Mehta NM, Corkins MR, Lyman B, et al. Defining pediatric malnutrition: a paradigm shift toward etiology-related definitions. JPEN J Parenter Enteral Nutr. 2013;37(4):460-481. 6. Goldberg DL, Van Poots, HA. Pediatric and Neonatal Malnutrition: A Collaborative, Family-Centered Approach Improves Outcomes. Pediatr Neonatal Nurs Open J. 2019;6(1):e1-e4. 7. Malnutrition. Johns Hopkins Medicine website. Accessed July 24, 2023. https://www.hopkinsmedicine.org/health/conditions-and-diseases/malnutrition 8. Symptoms Malnutrition. NHS website. Last reviewed May 23, 2023. Accessed July 24, 2023. https://www.nhs.uk/conditions/malnutrition/symptoms/ 9. Gambra-Arzoz M, Alonso-Cadenas JA, Jiménez-Legido M, et al. Nutrition Risk in Hospitalized Pediatric Patients: Higher Complication Rate and Higher Costs Related to Malnutrition. Nutr Clin Pract. 2020;35(1):157-163. 10. Malnutrition in children. World Health Organization website. Accessed July 26, 2023. https://www.who.int/data/nutrition/nlis/info/malnutrition-in-children#:~:text=Stuntingistheresultof,productivityatthenationallevel 11. What Is Parenteral Nutrition. ASPEN website. Accessed July 26, 2023. https://www.nutritioncare.org/About_Clinical_Nutrition/What_is_Parenteral_Nutrition/ 12. Lapillonne A, Fidler Mis N, Goulet O, et al. ESPGHAN/ESPEN/ESPR/CSPEN guidelines on pediatric parenteral nutrition: Lipids. Clin Nutr. 2018;37(6 Pt B):2324-2336. 13. Cober MP, Gura KM, Mirtallo JM, et al. ASPEN lipid injectable emulsion safety recommendations part 2: Neonate and pediatric considerations. Nutr Clin Pract. 2021;36(6):1106-1125.

Nutrition support for the critically ill is critical
How does malnutrition impact critically ill patients?
Several studies have observed a relationship between malnutrition and increased risk of mortality, infections, hospital length of stay.1-8 And unfortunately, many critically ill patients—those with cancer, Crohn’s disease, and short bowel syndrome just to name a few—often become malnourished. These conditions can affect their digestive system or interrupt their bowel function for a long time—or indefinitely. When this happens, clinical nutrition is needed to adequately nourish these patients.
More than 30% of patients in the intensive care unit have been reported to be malnourished.9
PN in critical care
Many critically ill patients cannot tolerate food by mouth or enteral feedings. In these cases, PN plays a crucial role in helping them meet their nutritional goals. In addition to amino acids, lipids, and dextrose, essential fatty acids (EFAs) are…well…essential to providing optimal nutrition.
The good news is that most lipid-based PN products provide EFAs. Lipid sources used in PN include soybean oil, medium-chain triglycerides (MCTs), olive oil, and fish oil. With SMOFlipid® (Lipid Injectable Emulsion, USP 20%), you get all four—each with a unique characteristic.
-

What makes SMOFlipid (Lipid Injectable Emulsion, USP 20%) unique?
SMOFlipid is the FIRST and ONLY four-oil lipid injectable emulsion (ILE) with demonstrated safety and tolerability10,11 in more than 7 million patients worldwide.* This unique combination of oils provides a balanced blend for critically ill patients of all ages—from adults to teenagers to preterm neonates.12
*Data on file.
What are the benefits of the blend?
-

Soybean oil 30% (omega-6)
Provides essential fatty acids.
-

Medium-chain triglycerides 30%
A source of rapidly available energy.13
-

Olive oil 25% (omega-9)
Supplies monounsaturated fatty acids.
-

Fish oil 15% (omega-3)
A source of EPA
and DHA.14
Studies showed triglyceride levels increased less with SMOFlipid compared to lipid emulsions with higher soybean oil content in adults.15,16
Triglyceride Change After 5 Days
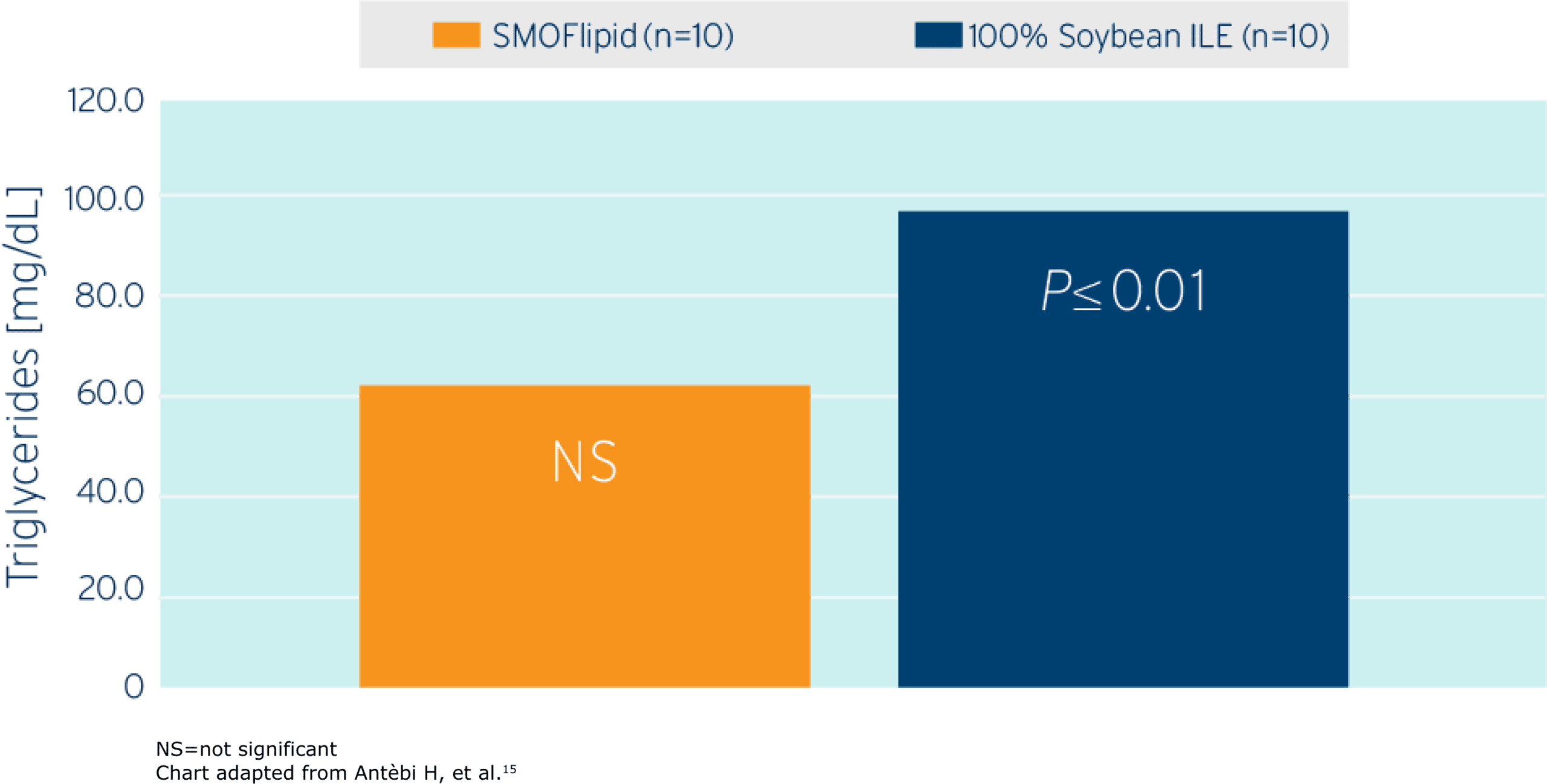
Study Takeaway
In this randomized controlled trial including postoperative surgical ICU adult patients (n=20), SMOFlipid demonstrated a lower triglyceride increase compared to those patients who received a lipid emulsion (1.5 g/kg/d ILE dose in both groups) with a higher soybean oil content.15
Monitor serum triglycerides before and during treatment with SMOFlipid. Company-sponsored studies showed that mean triglyceride levels from baseline values to week 4 were similar in both the SMOFlipid and comparator groups.
In pediatric patients, SMOFlipid has been shown to support growth and provides essential fatty acids.12,17,18
With SMOFlipid, Fresenius Kabi answered the call from PN and critical care medical societies for an alternative to soy-based ILEs.
As pioneers in clinical nutrition, Fresenius Kabi is committed to bringing innovations like SMOFlipid to market. Learn more about this unique 4-oil ILE and how it can help nourish your critically ill patients of any age at www.FreseniuKabiNutrition.com/products/smoflipid-adults/
SMOFLIPID (lipid injectable emulsion), for intravenous use
BRIEF SUMMARY OF PRESCRIBING INFORMATION
This brief summary does not include all the information needed to use SMOFlipid safely and effectively. Please see full prescribing information for intravenous use at www.FreseniusKabiNutrition.com/SMOFlipidPI.
INDICATIONS AND USAGE
SMOFlipid is indicated in adult and pediatric patients, including term and preterm neonates, as a source of calories and essential fatty acids for parenteral nutrition (PN) when oral or enteral nutrition is not possible, insufficient, or contraindicated.
DOSAGE AND ADMINISTRATION
The recommended daily dosage and initial and maximum infusion rates for pediatric and adult patients are provided in Table 1. Do not exceed the recommended maximum infusion rate in Table 1. The recommended duration of infusion for SMOFlipid will vary depending on the clinical situation. Adjust the administration flow rate by taking into account the dose being administered, the daily volume/intake, and the duration of the infusion.
SMOFlipid 1000 mL is supplied as a Pharmacy Bulk Package for admixing only and is not for direct infusion. Prior to administration, transfer to a separate PN container for individual patient use. Use a non-vented, non-DEHP 1.2 micron in-line filter during administration. Protect the admixed PN solution from light.
Table 1: Recommended Pediatric and Adult Dosage and Infusion Rate
| Age | Nutritional Requirements | Direct Infusion Rate | |
|---|---|---|---|
| Recommended Initial Dosage and Maximum Dosage | Initial | Maximum | |
| Birth to 2 years of age (including preterm and term neonates*) | Initial 0.5 to 1 g/kg/day not to exceed 3 g/kg/day** |
0.1 to 0.2 mL/kg/hour for the first 15 to 30 minutes; gradually increase to the required rate after 30 minutes | 0.75 mL/kg/hour |
| Pediatric patients 2 to <12 years of age | Initial 1 to 2 g/kg/day not to exceed 3 g/kg/day** |
0.2 to 0.4 mL/kg/hour for the first 15 to 30 minutes; gradually increase to the required rate after 30 minutes | 0.75 mL/kg/hour |
| Pediatric patients 12 to 17 years of age | Initial 1 g/kg/day not to exceed 2.5 g/kg/day** |
0.2 to 0.4 mL/kg/hour for the first 15 to 30 minutes; gradually increase to the required rate after 30 minutes | 0.75 mL/kg/hour |
| Adults | 1 to 2 g/kg/day not to exceed 2.5 g/kg/day** |
0.2 mL/kg/hour for the first 15 to 30 minutes; gradually increase to the required rate after 30 minutes | 0.5 mL/kg/hour |
*The neonatal period is defined as including term, post-term, and preterm newborn infants. The neonatal period for term and post-term infants is the day of birth plus 27 days. For preterm infants, the neonatal period is defined as the day of birth through the expected age of delivery plus 27 days (i.e., 44 weeks post-menstrual age).
** Daily dosage should not exceed a maximum of 60% of total energy requirements
CONTRAINDICATIONS
- Known hypersensitivity to fish, egg, soybean, peanut or to any of the active or inactive ingredients in SMOFlipid.
- Severe disorders of lipid metabolism characterized by hypertriglyceridemia (serum triglycerides >1,000 mg/dL).
WARNINGS AND PRECAUTIONS
-
Clinical Decompensation with Rapid Infusion of Intravenous Lipid Emulsion in Neonates and Infants.
In the postmarketing setting, serious adverse reactions including acute respiratory distress, metabolic acidosis, and death have been reported in neonates and infants after rapid infusion of intravenous lipid emulsions. Hypertriglyceridemia was commonly reported.
Strictly adhere to the recommended total daily dosage; the hourly infusion rate should not exceed 0.75 mL/kg/hour.
Preterm and small for gestational age infants have poor clearance of intravenous lipid emulsion and increased free fatty acid plasma levels following lipid emulsion infusion.
Carefully monitor the infant’s ability to eliminate the infused lipids from the circulation (e.g., measure serum triglycerides and/or plasma free fatty acid levels). If signs of poor clearance of lipids from the circulation occur, stop the infusion and initiate a medical evaluation.
-
Parenteral Nutrition-Associated Liver Disease and Other Hepatobiliary Disorders.
Risk of Parenteral Nutrition-Associated Liver Disease (PNALD): PNALD, or Intestinal failure-associated liver disease (IFALD), can present as cholestasis or hepatic stenosis and may progress to steatohepatitis with fibrosis and cirrhosis (possibly leading to chronic hepatic failure). The etiology of PNALD is multifactorial; however, intravenously administered phytosterols (plant sterols) contained in plant-derived lipid emulsions, including SMOFlipid, have been associated with development of PNALD.
In a randomized study of neonates and infants expected to be treated with PN for at least 28 days, parenteral nutrition-associated cholestasis (PNAC), a precursor to PNALD, developed less frequently in SMOFlipid-treated patients than in 100% soybean oil lipid emulsion-treated patients.
Monitor liver tests in patients treated with SMOFlipid and consider discontinuation or dosage reduction if abnormalities occur.
Other Hepatobiliary Disorders
Hepatobiliary disorders including cholecystitis and cholelithiasis have developed in some parenteral nutrition-treated patients without preexisting liver disease. Monitor liver tests when administering SMOFlipid. Patients developing signs of hepatobiliary disorders should be assessed early to determine whether these conditions are related to SMOFlipid use.
- Hypersensitivity Reactions: SMOFlipid contains soybean oil, fish oil, and egg phospholipids, which may cause hypersensitivity reactions. Cross reactions have been observed between soybean and peanut. SMOFlipid is contraindicated in patients with known hypersensitivity to fish, egg, soybean, peanut, or any of the active or inactive ingredients in SMOFlipid. If a hypersensitivity reaction occurs, stop infusion of SMOFlipid immediately and initiate appropriate treatment and supportive measures.
- Infections: Lipid emulsions, such as SMOFlipid, can support microbial growth and are an independent risk factor for the development of catheter-related bloodstream infections. To decrease the risk of infectious complications, ensure aseptic techniques are used for catheter placement, catheter maintenance, and preparation and administration of SMOFlipid. Monitor for signs and symptoms of infection including fever and chills, as well as laboratory test results that might indicate infection (including leukocytosis and hyperglycemia). Perform frequent checks of the intravenous catheter insertion site for edema, redness, and discharge.
-
Fat Overload Syndrome: This is a rare condition that has been reported with intravenous lipid emulsions and is characterized by a sudden deterioration in the patient’s condition (e.g., fever, anemia, leukopenia, thrombocytopenia, coagulation disorders, hyperlipidemia, hepatomegaly, deteriorating liver function, and central nervous system manifestations such as coma). A reduced or limited ability to metabolize lipids, accompanied by prolonged plasma clearance (resulting in higher lipid levels), may result in this syndrome. Although fat overload syndrome has been most frequently observed when the recommended lipid dose or infusion rate was exceeded, cases have also been described when the lipid formulation was administered according to instructions.
If signs or symptoms of fat overload syndrome occur, stop SMOFlipid. The syndrome is usually reversible when the infusion of the lipid emulsion is stopped.
- Refeeding Syndrome: Administering PN to severely malnourished patients may result in refeeding syndrome, which is characterized by the intracellular shift of potassium, phosphorus, and magnesium as patients become anabolic. Thiamine deficiency and fluid retention may also develop. To prevent these complications, closely monitor severely malnourished patients and slowly increase their nutrient intake.
-
Hypertriglyceridemia: The use of SMOFlipid is contraindicated in patients with hypertriglyceridemia with serum triglyceride concentrations >1,000 mg/dL.
Patients with conditions such as inherited lipid disorders, obesity, diabetes mellitus, or metabolic syndromes have a higher risk of developing hypertriglyceridemia with the use of SMOFlipid. In addition, patients with hypertriglyceridemia may have worsening of their hypertriglyceridemia with administration of SMOFlipid. Excessive dextrose administration may further increase such risk.
Evaluate patients’ capacity to metabolize and eliminate the infused lipid emulsion by measuring serum triglycerides before the start of infusion (baseline value) and regularly throughout treatment. If triglyceride levels are above 400 mg/dL in adults, stop the SMOFlipid infusion and monitor serum triglyceride levels to avoid clinical consequences of hypertriglyceridemia such as pancreatitis. In pediatric patients with hypertriglyceridemia, lower triglyceride levels (i.e., below 400 mg/dL) may be associated with adverse reactions. Monitor serum triglyceride levels to avoid potential complications with hypertriglyceridemia such as pancreatitis, lipid pneumonitis, and neurologic changes, including kernicterus.
To minimize the risk of new or worsening of hypertriglyceridemia, assess high-risk patients for their overall energy intake including other sources of lipids and dextrose, as well as concomitant drugs that may affect lipid and dextrose metabolism.
- Aluminum Toxicity: SMOFlipid contains no more than 25 mcg/L of aluminum. Prolonged PN administration in patients with renal impairment may result in aluminum reaching toxic levels. Preterm infants are at greater risk because their kidneys are immature, and they require large amounts of calcium and phosphate solutions, which contain aluminum. Patients with impaired kidney function, including preterm infants, who receive parenteral levels of aluminum at greater than 4 to 5 mcg/kg/day can accumulate aluminum at levels associated with central nervous system and bone toxicity. Tissue loading may occur at even lower rates of administration.
- Essential Fatty Acid Deficiency: Treatment-emergent cases of moderate or severe essential fatty acid deficiency (EFAD) (defined as the triene [Mead acid] to tetraene [arachidonic acid] ratio >0.2 and >0.4, respectively) were not observed in pediatric clinical trials of SMOFlipid up to 28 days. However, cases of EFAD have been reported in adults and pediatric patients in the postmarketing period with the use of SMOFlipid. The median time to onset was greater than 28 days among cases that reported time to onset. Monitor patients for laboratory evidence (e.g., abnormal fatty acid levels) and clinical symptoms of EFAD (e.g., skin manifestations and poor growth) because these signs may emerge before laboratory evidence of EFAD is confirmed. Laboratory testing using the triene to tetraene ratio may not be adequate to diagnose EFAD, and assessment of individual fatty acid levels may be needed. Ensure patients are receiving recommended dosages of SMOFlipid to prevent EFAD.
-
Monitoring/Laboratory Tests: Throughout treatment monitor serum triglycerides, fluid and electrolyte status, blood glucose, liver and kidney function, coagulation parameters, and complete blood count including platelets.
The lipids contained in SMOFlipid may interfere with some laboratory blood tests (e.g., hemoglobin, lactate dehydrogenase [LDH], bilirubin, and oxygen saturation) if blood is sampled before lipids have cleared from the bloodstream. Conduct these blood tests at least 6 hours after stopping the infusion. SMOFlipid contains vitamin K that may counteract anticoagulant activity.
ADVERSE REACTIONS
Most common adverse drug reactions >1% of adult patients who received SMOFlipid from clinical trials were nausea, vomiting, hyperglycemia, flatulence, pyrexia, abdominal pain, increased blood triglycerides, hypertension, sepsis, dyspepsia, urinary tract infection, anemia, and device-related infection.
Less common adverse reactions in ≤1% of adult patients who received SMOFlipid were dyspnea, leukocytosis, diarrhea, pneumonia, cholestasis, dysgeusia, increased blood alkaline phosphatase, increased gamma-glutamyltransferase, increased C-reactive protein, tachycardia, liver function test abnormalities, headache, pruritis, dizziness, rash, and thrombophlebitis.
The most common adverse drug reactions in >1% of pediatric patients who received SMOFlipid were anemia, vomiting, gamma-glutamyltransferase increased, nosocomial infection, cholestasis, pyrexia, C-reactive protein increased, hyperbilirubinemia, abdominal pain, bilirubin conjugated increased, diarrhea, tachycardia, thrombocytopenia, hyperglycemia, and sepsis.
Less common adverse reactions in ≤1% of pediatric patients who received SMOFlipid were decreased hematocrit, metabolic acidosis, increased blood triglycerides, infection, increased blood alkaline phosphatase, increased alanine aminotransferase, fluid overload, hypertension, hypertriglyceridemia, and rash.
The following adverse reactions have been identified during post-approval use of SMOFlipid in countries where it is registered. Cardiac disorders: palpitations; General disorders and administration site conditions: chills, chest pain, malaise; Hepatobiliary disorders: cholestasis; Infections and infestations: infection; Metabolism and nutrition disorders: fatty acid deficiency; Respiratory, thoracic and mediastinal disorders: dyspnea; Skin and subcutaneous tissue disorders: hyperhidrosis; Vascular disorders: phlebitis.
To report SUSPECTED ADVERSE REACTIONS, contact Fresenius Kabi USA, LLC at 1-800-551-7176, option 5, or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
Soybean and olive oils in SMOFlipid contain vitamin K1 which may counteract the anticoagulant activity of vitamin K antagonists such as warfarin. In patients who receive concomitant SMOFlipid and warfarin, increase monitoring of laboratory parameters for anticoagulant activity.
USE IN SPECIFIC POPULATIONS
- Pregnancy and Lactation: Administration of the recommended dose of SMOFlipid is not expected to cause major birth defects, miscarriage, or other adverse maternal or fetal outcomes. No animal reproduction studies have been conducted with SMOFlipid. Administration of the recommended dose of SMOFlipid is not expected to cause harm to a breastfed infant. There are no data on the presence of SMOFlipid in human or animal milk or its effects on milk production.
- Pediatric Use: The safety and effectiveness of SMOFlipid have been established as a source of calories and essential fatty acids for parenteral nutrition when oral or enteral nutrition is not possible, insufficient, or contraindicated in pediatric patients, including term and preterm neonates. Use of SMOFlipid in neonates is supported by evidence from short-term (i.e., 1- to 4-week) studies, and one study following neonates beyond 4 weeks. Use of SMOFlipid in older pediatric patients is supported by evidence from a short-term (i.e., <28 days) study in pediatric patients 28 days to 12 years of age and additional evidence from studies in adults. The most common adverse reactions in SMOFlipid-treated pediatric patients were anemia, vomiting, gamma-glutamyltransferase increased, and nosocomial infection. PNALD, also referred to as IFALD, has been reported in pediatric patients who received SMOFlipid for more than 2 weeks. PNAC (a precursor to PNALD) was reported less frequently in SMOFlipid-treated patients compared to soybean oil lipid emulsion-treated patients in Pediatric Study 1. Although clinically significant cases of EFAD were not observed during short-term use in pediatric clinical studies, cases of EFAD have been reported with the use of SMOFlipid in the postmarketing setting. Monitor pediatric patients for laboratory evidence of EFAD because they may be particularly vulnerable to neurologic complications if adequate amounts of essential fatty acids are not provided. In the post marketing setting, clinical decompensation with rapid infusion of intravenous lipid emulsion in neonates and infants, sometimes fatal has been reported. Because of immature renal function, preterm infants receiving prolonged treatment with SMOFlipid may be at risk for aluminum toxicity.
- Geriatric Use: Energy expenditure and requirements may be lower for older adults than younger patients. Of the 354 adult patients in clinical studies of SMOFlipid, 35% were >65 years of age and 10% were >75 years of age. No overall differences in the safety and efficacy of SMOFlipid were observed between these patients and younger patients, and other reported clinical experience has not identified differences in responses between the elderly and younger patients, but greater sensitivity in some older patients cannot be ruled out.
OVERDOSAGE
In the event of an overdose, serious adverse reactions may result. Stop the SMOFlipid infusion until triglyceride levels have normalized and symptoms have abated. The effects are usually reversible by stopping the lipid infusion. If medically appropriate, further intervention may be indicated. Lipids are not dialyzable from plasma.
3673-SMF-08-06/23