

Navigating lipid sources in parenteral nutrition (PN)
Because lipid injectable emulsions (ILEs) are an energy-dense source of calories in PN solutions, choosing a lipid source is important. In this blog post, we’ll discuss various lipid sources and compare 2 mixed-oil ILEs.
Evaluating lipid sources: A variety of lipid sources are available for PN, including medium-chain triglycerides (MCTs), olive oils, and fish oils, which, based on extensive usage in Europe, have an equivalent safety profile to soybean oil.1 These alternative ILEs are metabolized via different pathways.1
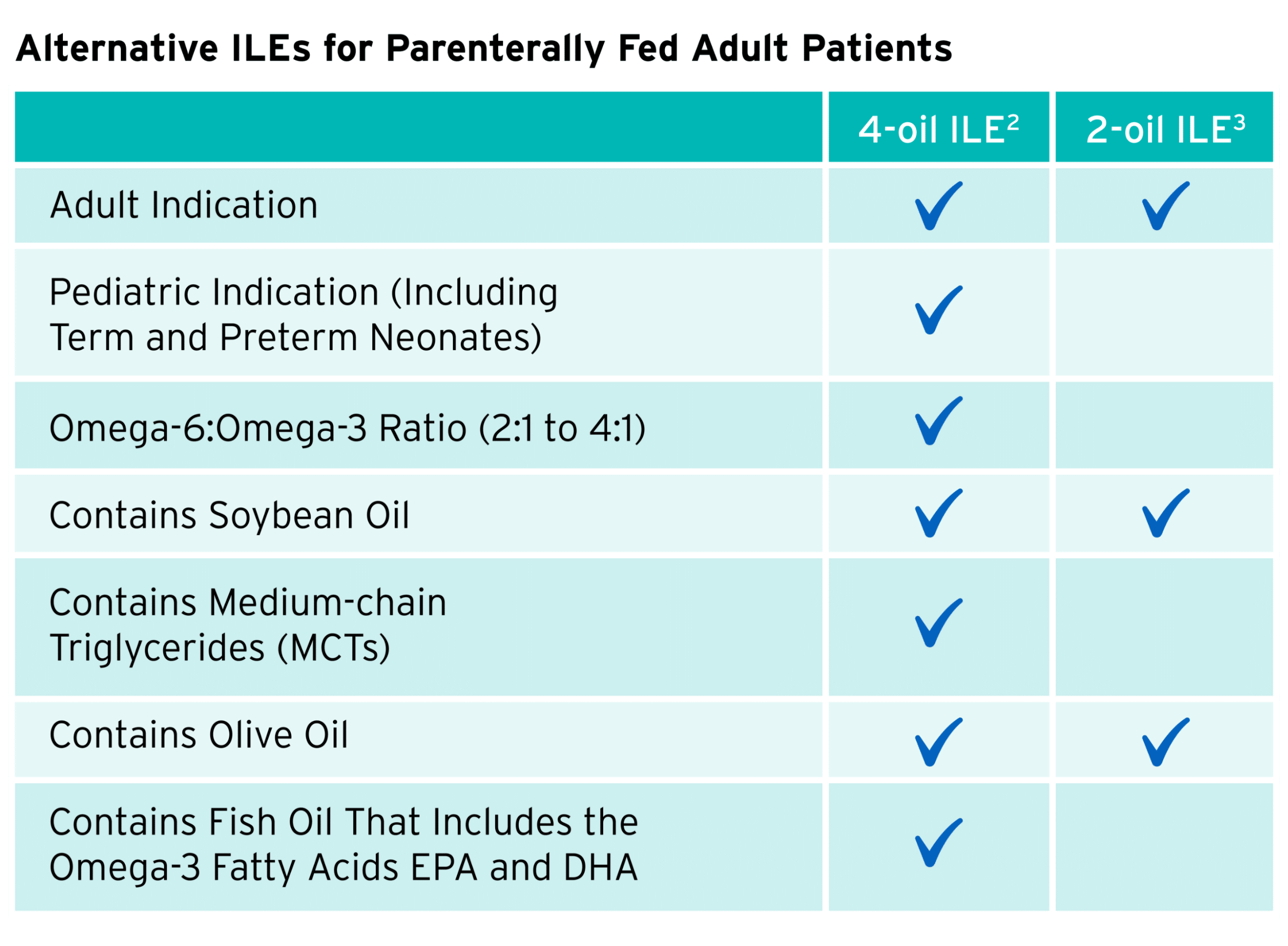
Mixed-oil ILEs: While standard soybean oil-based emulsions are commonly used, alternative mixed-oil emulsions containing omega-3s and omega-6s can be a unique blend due to their diverse fatty acid content.
Tailoring nutrition: Recognizing that each patient is unique, it is important to take into consideration the patient’s age, clinical condition, therapeutic objectives, and tolerance when considering a lipid dose.
The role of clinical expertise: The expertise of healthcare professionals is paramount in the selection and management of lipid sources in PN. Ongoing assessment and monitoring of patients is integral to ensure the appropriate choice of lipid for each patient.
An informed approach is critical to incorporating mixed-oil ILEs as a part of the clinical management of patients. Alternative lipid sources containing fish oil are the 4th generation of ILEs. Specifically, a 4-oil ILE was the most recent innovation to the market, receiving approval for adults in 2016 and pediatrics in 2022.
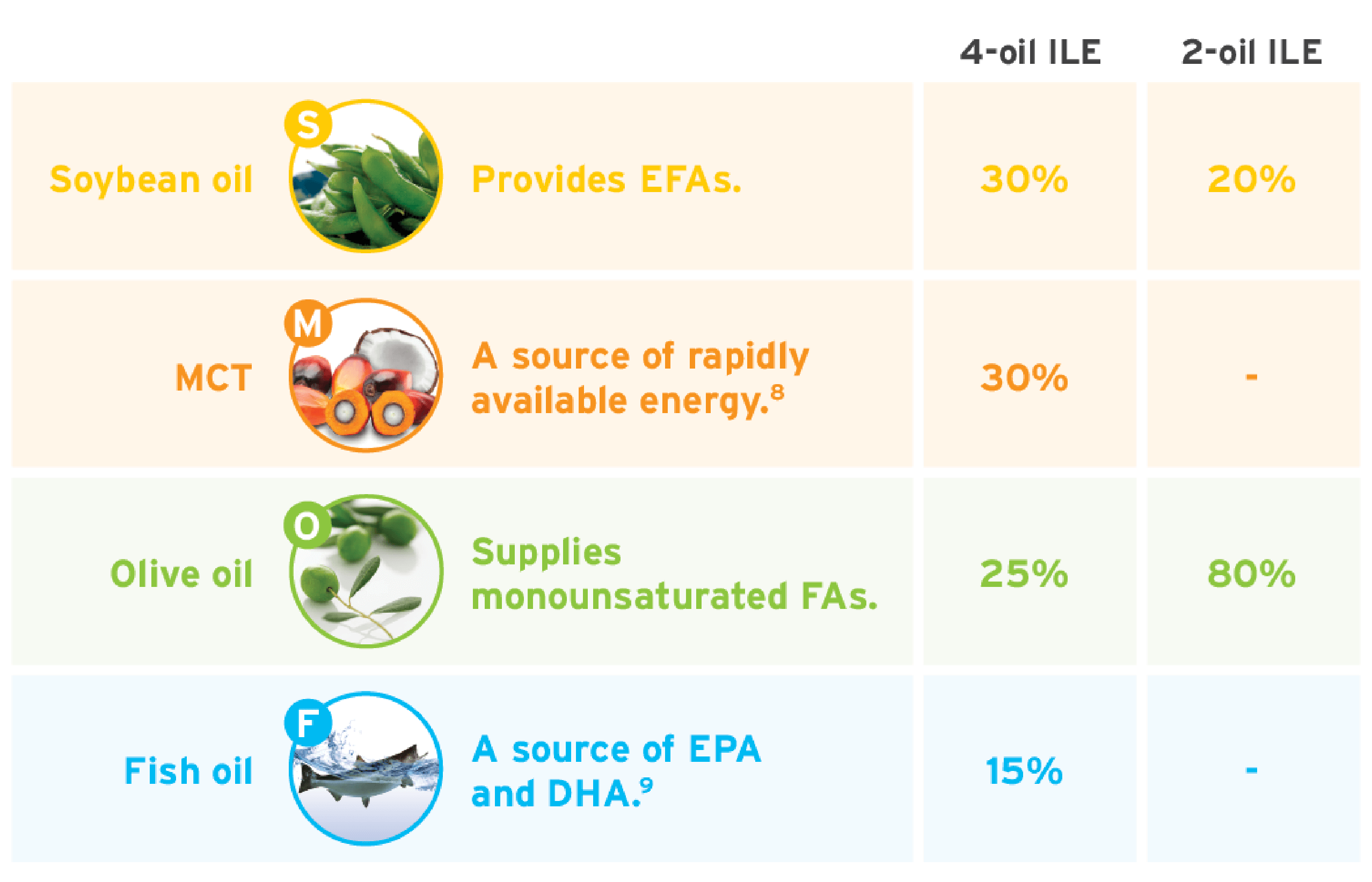
Use this table as a quick reference on the compositional differences between 2 mixed-oil ILEs on the market today.
Expert recommendations suggest an omega-6:omega-3 ratio of 2:1 to 4:1 in ILEs.4-7
At Fresenius Kabi, our dedication to caring also means we’re dedicated to keeping healthcare professionals informed. Staying current with the latest research and news in the field of PN can help you educate patients about the treatments they receive, including the importance of PN and the specific products being used.
Looking for more information?
SMOFlipid® (lipid injectable emulsion, USP), for intravenous use
IMPORTANT SAFETY INFORMATION
What is SMOFlipid?
- Indicated in adult and pediatric patients as a source of calories and essential fatty acids for parenteral nutrition (PN) when oral or enteral nutrition is not possible, insufficient, or contraindicated.
- The hourly infusion rate in pediatrics should not exceed 0.75 mL/kg/hour and 0.5 mL/kg/hour in adults.
SMOFlipid should not be received by patients who have:
- A known allergy to fish, egg, soybean, or peanut, or to any of the active or inactive ingredients in SMOFlipid.
- Abnormally high levels of lipid (triglycerides) in the blood.
SMOFlipid may cause serious side effects including:
- Serious Adverse Reactions with Rapid Infusion of Intravenous Lipid Emulsion in Neonates and Infants: Strictly follow the recommended total daily dosage and do not exceed the maximum infusion rate. If poor clearance of fats occurs, the infusion should be stopped, and a medical evaluation started.
- Risk of Parenteral Nutrition-Associated Liver Disease: Parenteral nutrition-associated liver disease (PNALD) may progress to liver inflammation and damage caused by a buildup of fat in the liver with scarring and cirrhosis.
- Allergic Reactions: Contact your healthcare provider immediately if you are experiencing an allergic reaction.
- Fat Overload Syndrome, Refeeding Syndrome, Elevated Triglycerides (Hypertriglyceridemia): Your healthcare provider will monitor you for signs and symptoms of early infection and blood levels.
Monitoring/Laboratory Tests: The content of vitamin K may interfere with blood clotting activity of medications.
The most common side effects (>1%) in adult patients include nausea, vomiting, and high levels of glucose in the blood and in pediatric patients include low levels of red blood cells, vomiting, increased levels of liver enzymes (i.e., gamma-glutamyltransferase) and hospital-acquired infections.
These are not all the possible side effects associated with SMOFlipid. Call your healthcare provider for medical advice regarding SMOFlipid side effects. You are encouraged to report negative side effects of SMOFlipid. Contact Fresenius Kabi USA, LLC at: 1-800-551-7176 or FDA at: 1-800-FDA-1088 or www.fda.gov/medwatch. The FDA-approved product labeling can be found at https://www.freseniuskabinutrition.com/SMOFlipidPI.
