
Nutrition in NEC: what are your options?
Babies who are born too soon are vulnerable to the development of serious health conditions like necrotizing enterocolitis (NEC), an intestinal disease that affects babies during their first weeks and months of life.1,2 It’s a complicated disease in which the tissues of the intestines become necrotic, potentially leading to peritonitis, sepsis, and even death.3
“NEC affects 2% to 5% of all premature infants and is responsible for nearly 8% of all NICU admissions.”3
What causes NEC?
When the intestinal lining is damaged and torn, the bacteria that’s inside the intestine can enter the peritoneum and bloodstream, resulting in infection.2,3 Preemies, very low birth weight babies, and formula-fed babies are especially at risk for developing NEC; however, the disease can also affect full-term babies, particularly those who are born with a hypoxic medical condition such as a cyanotic congenital heart defect.3
While many babies fully recover from the disease without any further complications, some may face long-term challenges with cognition, muscle function, and intestinal function.3,4 And unfortunately, death can occur in 10-50% of babies who develop this devastating disease.1,3
What are the signs that a baby may have NEC?
A baby may present with the following nonspecific signs and symptoms3:
- Swollen belly
- Decreased activity
- Appetite loss
- Vomiting
- Diarrhea
- Bloody stools
As NEC gets worse, a baby can experience respiratory and circulatory problems and may even become unresponsive.3
How is NEC treated?
Treatment of NEC involves stopping all feedings by mouth and maintaining NPO status, or “nothing by mouth.”3 A nasogastric tube is placed to decompress the dilated bowels and intravenous (IV) broad-spectrum antibiotics are started.3 Some babies may require surgical removal of the affected portion of the intestine.3
When babies with NEC are unable to receive nutrition by mouth, parenteral nutrition (PN) should be initiated to help nourish these vulnerable patients.3
Nourishing tiny patients with PN
As the U.S. market leader in lipid injectable emulsions (ILEs),* we have two solutions that are approved for use in even the youngest PN patients to help them meet their nutritional needs. What’s unique about these two solutions is that they both contain fish oil that provides omega-3 fatty acids.
Our FIRST and ONLY 4-oil lipid injectable emulsion (ILE), SMOFlipid, has demonstrated safety and tolerability5,6 in more than 7 million adult and pediatric patients worldwide.* SMOFlipid is the only PN innovation with a unique blend of 4 oils to nourish patients of all ages, including the smallest patients.7
SMOFlipid helps to nurture growth in pediatric patients.7-10
Parenteral nutrition-associated cholestasis (PNAC) is a significant complication that develops among pediatric patients who require long-term PN,11 which can be the case for some patients who have NEC.3 In addition, premature birth, low birth weight, intestinal failure secondary to NEC, and active recovery from surgical NEC can increase a baby’s risk for developing PNAC.11-15
Omegaven is the FIRST and ONLY PN innovation with 100% fish oil that nurtures pediatric patients with PNAC.16 It was previously available only for compassionate care, but Fresenius Kabi saw the need for the product to be more broadly available to pediatric patients and worked hard to secure FDA approval in 2018.
The omega-3 fatty acids EPA and DHA are considered to be important for the healthy development of infants due to their special physiological roles.17,18
A tireless dedication to driving advancements in clinical nutrition has allowed us to deliver more options to you and your most delicate PN patients. We will continue to build on our history of innovation in clinical nutrition to further the field for all patients on PN. Learn more about our innovations that nourish pediatric patients at www.FreseniusKabiNutrition.com/pn-portfolio/.
INDICATIONS AND USAGE
Omegaven is indicated as a source of calories and fatty acids in pediatric patients with parenteral nutrition-associated cholestasis (PNAC).
Limitations of Use
Omegaven is not indicated for the prevention of PNAC. It has not been demonstrated that Omegaven prevents PNAC in parenteral nutrition (PN)-dependent patients.
It has not been demonstrated that the clinical outcomes observed in patients treated with Omegaven are a result of the omega-6: omega-3 fatty acid ratio of the product.
IMPORTANT SAFETY INFORMATION
Protect the admixed PN solution from light. Prior to administration, correct severe fluid and electrolyte disorders and measure serum triglycerides to establish a baseline level. Initiate dosing in PN-dependent pediatric patients as soon as direct or conjugated bilirubin levels are 2 mg/dL or greater. The recommended daily dose (and the maximum dose) in pediatric patients is 1 g/kg/day. Administer Omegaven until direct or conjugated bilirubin levels are less than 2 mg/dL or until the patient no longer requires PN.
Omegaven is contraindicated in patients with known hypersensitivity to fish or egg protein or to any of the active ingredients or excipients, severe hemorrhagic disorders due to a potential effect on platelet aggregation, severe hyperlipidemia or severe disorders of lipid metabolism characterized by hypertriglyceridemia (serum triglyceride concentrations greater than 1,000 mg/dL).
Risk of Death in Preterm Infants due to Pulmonary Lipid Accumulation: Deaths in preterm infants after infusion of soybean oil-based intravenous lipid emulsions have been reported in medical literature. Autopsy findings in these preterm infants included intravascular lipid accumulation in the lungs. The risk of pulmonary lipid accumulation with Omegaven is unknown. Preterm and small-for-gestational-age infants have poor clearance of intravenous lipid emulsion and increased free fatty acid plasma levels following lipid emulsion infusion. This risk due to poor lipid clearance should be considered when administering intravenous lipid emulsions. Monitor patients receiving Omegaven for signs and symptoms of pleural or pericardial effusion.
Hypersensitivity Reactions: Monitor for signs or symptoms. Discontinue infusion if reaction occurs.
Risk of Infections, Fat Overload Syndrome, Refeeding Syndrome, and Hypertriglyceridemia: Monitor for signs and symptoms; monitor laboratory parameters.
Aluminum Toxicity: Increased risk in patients with renal impairment, including preterm infants.
Monitoring and Laboratory Tests: Routine laboratory monitoring is recommended, including monitoring for essential fatty acid deficiency.
The most common adverse drug reactions (>15%) are: vomiting, agitation, bradycardia, apnea and viral infection.
To report SUSPECTED ADVERSE REACTIONS, contact Fresenius Kabi USA, LLC at 1-800-551-7176, option 5, or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
This Important Safety Information does not include all the information needed to use Omegaven safely and effectively. Please see full prescribing information for Omegaven (fish oil triglycerides) injectable emulsion for intravenous use at www.freseniuskabinutrition.com/OmegavenPI.
INDICATIONS AND USAGE
SMOFlipid is indicated in adult and pediatric patients, including term and preterm neonates, as a source of calories and essential fatty acids for parenteral nutrition (PN) when oral or enteral nutrition is not possible, insufficient, or contraindicated.
IMPORTANT SAFETY INFORMATION
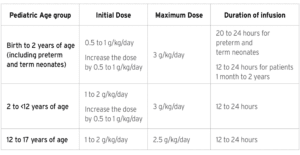
For intravenous infusion only into a central or peripheral vein. Use a non-vented non-DEHP 1.2 micron in-line filter set during administration. Recommended dosage depends on age, energy expenditure, clinical status, body weight, tolerance, ability to metabolize and eliminate lipids, and consideration of additional energy given to the patient. The recommended daily dosage in adults is 1 to 2 grams/kg per day and should not exceed 2.5 grams/kg per day. The recommended dose for pediatrics is shown in Table 1, and do not exceed an infusion rate of 0.15 g/kg/hour. SMOFlipid Pharmacy Bulk Package is only indicated for use in pharmacy admixture programs for the preparation of three-in-one or total nutrition admixtures. Protect the admixed PN solution from light.
Table 1: Recommended Pediatric Dosage
SMOFlipid is contraindicated in patients with known hypersensitivity to fish, egg, soybean, or peanut protein, or to any of the active ingredients or inactive ingredients, and severe disorders of lipid metabolism characterized by hypertriglyceridemia (serum triglycerides > 1,000 mg/dL).
Parenteral Nutrition-Associated Liver Disease: Increased risk in patients who received parenteral nutrition for greater than 2 weeks, especially preterm neonates. Monitor liver tests, if abnormalities occur consider discontinuation or dosage reduction.
Risk of Death in Preterm Infants due to Pulmonary Lipid Accumulation: Deaths in preterm infants after infusion of intravenous 100% soybean oil lipid emulsions have been reported in the literature.
Hypersensitivity Reactions: Monitor for signs or symptoms. Discontinue infusion if reactions occur.
Risk of Infections, Fat Overload Syndrome, Refeeding Syndrome, Hypertriglyceridemia, and Essential Fatty Acid Deficiency: Monitor for signs and symptoms; monitor laboratory parameters.
Aluminum Toxicity: Increased risk in patients with renal impairment, including preterm neonates.
Most common adverse drug reactions (≥5%) from clinical trials in adults were nausea, vomiting, and hyperglycemia. Most common adverse drug reactions (≥5%) from clinical trials in pediatric patients were anemia, vomiting, increased gamma-glutamyltransferase and nosocomial infection.
To report SUSPECTED ADVERSE REACTIONS, contact Fresenius Kabi USA, LLC at 1-800-551-7176, option 5, or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
This Important Safety Information does not include all the information needed to use SMOFlipid safely and effectively. Please see full prescribing information, for intravenous use at www.freseniuskabinutrition.com/SMOFlipidPI.
4127-OMEG-08-05/23