

Omega-3s in parenteral nutrition (PN)
The role of lipids in PN
People of all ages, from infants to children to adults, may need PN to help them meet their nutritional goals.1 Protein, carbohydrate, and fat are the 3 main components of PN. These 3 macronutrients are important for the provision of adequate nutrition in patients receiving PN.
Intravenous fats, or lipids, provide a good source of energy-dense, non-protein calories, which may lower the amount of carbohydrate needed as part of nutrition support.2,3
“Lipids provide the building blocks for cell membranes and provide essential fatty acids, thereby preventing essential fatty acid deficiency.”3
Lipid source matters when choosing appropriate lipid injectable emulsions (ILEs) for patients on PN. Fish oil-containing ILEs are a source of omega-3 fatty acids. Let’s dive further into some omega-3 properties and explore innovative PN products that contain this alternative energy source.
Introducing an advancement in PN
Fresenius Kabi is dedicated to delivering more options to healthcare providers and their PN patients. So, in 2016, we introduced fish oil and omega-3s into our PN products, representing a development and advancement for critically ill patients.
Meet our innovative omega-3-containing PN products
We understand that lipid source matters when considering appropriate nutrition for patients. We’re proud to offer two innovative ILEs that deliver fish oil and omega-3s to help nourish patients who need PN. Meet SMOFlipid® (Lipid Injectable Emulsion, USP 20%) and Omegaven® (fish oil triglycerides) injectable emulsion.
With SMOFlipid, Fresenius Kabi answered the call from PN and critical care medical societies for an alternative to soy-based ILEs. This unique blend of 4 oils—soybean oil, medium-chain triglycerides, olive oil, and fish oil—is the first lipid advancement for adults in more than 4 decades. In 2022, it received approval for use in pediatric patients, including term and preterm neonates.4 Today, SMOFlipid is recognized all around the world.
-
Used for more than a decade worldwide
-
Administered to more than 7 million adult and pediatric patients worldwide*
-

Safe and well tolerated5,6
-

The fatty acid ratio is in line with expert recommendations in adults7-10
-

Contains fish oil, which provides a good source of omega-3 fatty acids, including eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA)11
Omegaven was previously available only for compassionate care. However, Fresenius Kabi saw the need for the product to be more broadly available to pediatric patients and worked hard to secure the necessary clinical evidence to support FDA approval. In 2018, Omegaven became commercially available for pediatric patients with parenteral nutrition-associated cholestasis (PNAC). It’s the FIRST and ONLY PN innovation with 100% fish oil that nurtures.12
-
Patients receiving Omegaven achieved age-appropriate growth12
-

Omegaven-treated patients experienced improvement in liver function parameters12
-
Direct or conjugated bilirubin levels were effectively lowered12
-

Contains the omega-3 fatty acids EPA and DHA
The development of these two products demonstrates our dedication to caring for life. And we will continue to drive advancements for all patients who need PN.
Learn more about SMOFlipid and Omegaven at www.FreseniusKabiNutrition.com/pn-portfolio/.
INDICATIONS AND USAGE
Omegaven is indicated as a source of calories and fatty acids in pediatric patients with parenteral nutrition-associated cholestasis (PNAC).
Limitations of Use
Omegaven is not indicated for the prevention of PNAC. It has not been demonstrated that Omegaven prevents PNAC in parenteral nutrition (PN)-dependent patients.
It has not been demonstrated that the clinical outcomes observed in patients treated with Omegaven are a result of the omega-6: omega-3 fatty acid ratio of the product.
IMPORTANT SAFETY INFORMATION
Protect the admixed PN solution from light. Prior to administration, correct severe fluid and electrolyte disorders and measure serum triglycerides to establish a baseline level. Initiate dosing in PN-dependent pediatric patients as soon as direct or conjugated bilirubin levels are 2 mg/dL or greater. The recommended nutritional requirements of fat and recommended dosages of Omegaven to meet those requirements for pediatric patients are provided in Table 1, along with recommendations for the initial and maximum infusion rates. Administer Omegaven until direct or conjugated bilirubin levels are less than 2 mg/dL or until the patient no longer requires PN.
Table 1: Recommended Pediatric Dosage and Infusion Rate

Omegaven is contraindicated in patients with known hypersensitivity to fish or egg protein or to any of the active ingredients or excipients, severe hemorrhagic disorders due to a potential effect on platelet aggregation, severe hyperlipidemia or severe disorders of lipid metabolism characterized by hypertriglyceridemia (serum triglyceride concentrations greater than 1,000 mg/dL).
Clinical Decompensation with Rapid Infusion of Lipid Injectable Emulsions in Neonates and Infants: Acute respiratory distress, metabolic acidosis, and death after rapid infusion of intravenous lipid emulsions have been reported. Hypertriglyceridemia was commonly reported. Strictly adhere to the recommended total daily dosage; the hourly infusion rate should not exceed 1.5 mL/kg/hour. Carefully monitor the infant’s ability to eliminate the infused lipids from the circulation (e.g., measure serum triglycerides and/or plasma free fatty acid levels). If signs of poor clearance of lipids from the circulation occur, stop the infusion and initiate a medical evaluation.
Hypersensitivity Reactions: Monitor for signs or symptoms. Discontinue infusion if reaction occurs.
Infections, Fat Overload Syndrome, Refeeding Syndrome, and Hypertriglyceridemia: Monitor for signs and symptoms; monitor laboratory parameters.
Aluminum Toxicity: Increased risk in patients with renal impairment, including preterm infants.
Monitoring and Laboratory Tests: Routine laboratory monitoring is recommended, including monitoring for essential fatty acid deficiency.
The most common adverse drug reactions (>15%) are: vomiting, agitation, bradycardia, apnea and viral infection.
To report SUSPECTED ADVERSE REACTIONS, contact Fresenius Kabi USA, LLC at 1-800-551-7176, option 5, or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
This Important Safety Information does not include all the information needed to use Omegaven safely and effectively. Please see full prescribing information for Omegaven (fish oil triglycerides) injectable emulsion for intravenous use at www.FreseniusKabiNutrition.com/OmegavenPI.
INDICATIONS AND USAGE
SMOFlipid is indicated in adult and pediatric patients, including term and preterm neonates, as a source of calories and essential fatty acids for parenteral nutrition (PN) when oral or enteral nutrition is not possible, insufficient, or contraindicated.
IMPORTANT SAFETY INFORMATION
For intravenous infusion only into a central or peripheral vein. Use a non-vented non-DEHP 1.2 micron in-line filter set during administration. Recommended dosage depends on age, energy expenditure, clinical status, body weight, tolerance, ability to metabolize and eliminate lipids, and consideration of additional energy given to the patient. The recommended dose for adults and pediatrics is shown in Table 1. For information on age-appropriate infusion rate, see the full prescribing information. SMOFlipid Pharmacy Bulk Package is only indicated for use in pharmacy admixture programs for the preparation of three-in-one or total nutrition admixtures. Protect the admixed PN solution from light.
Table 1: Recommended Adult and Pediatric Dosage
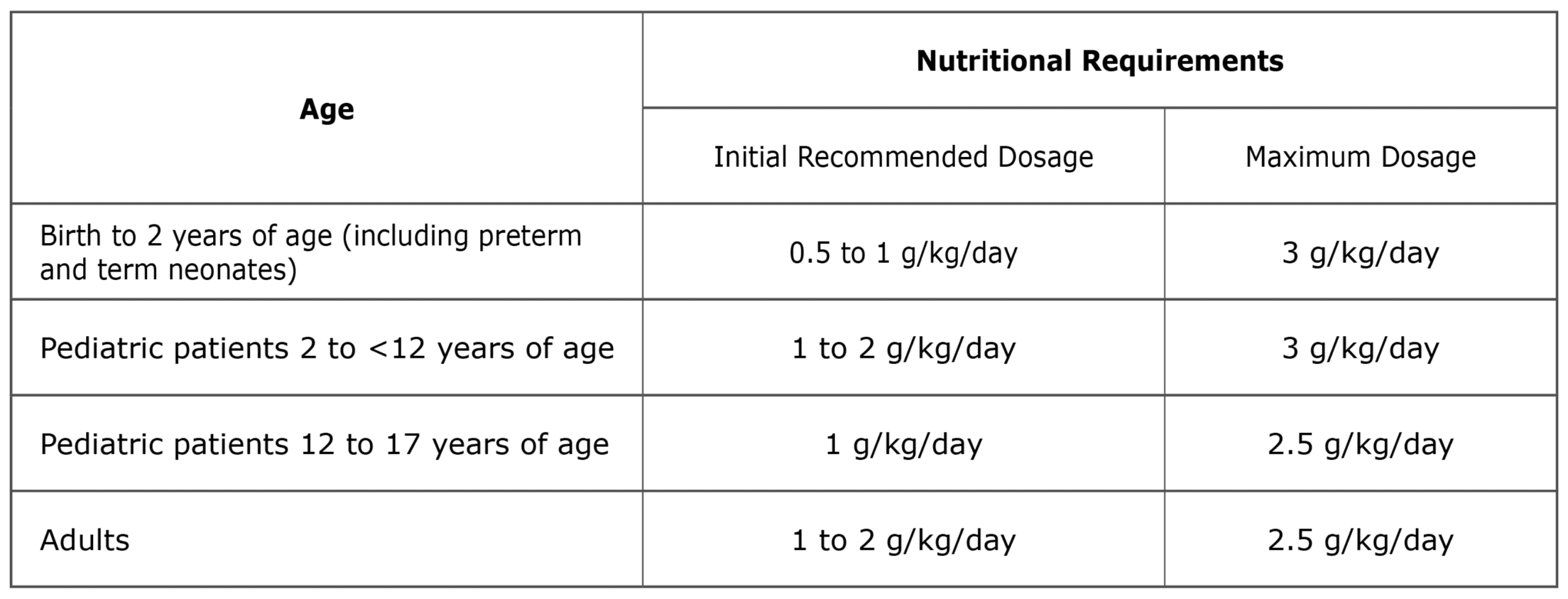
SMOFlipid is contraindicated in patients with known hypersensitivity to fish, egg, soybean, peanut, or any of the active or inactive ingredients, and severe disorders of lipid metabolism characterized by hypertriglyceridemia (serum triglycerides >1,000 mg/dL).
Clinical Decompensation with Rapid Infusion of Intravenous Lipid Emulsion in Neonates and Infants: Acute respiratory distress, metabolic acidosis, and death after rapid infusion of intravenous lipid emulsions have been reported.
Parenteral Nutrition-Associated Liver Disease: Increased risk in patients who received parenteral nutrition for greater than 2 weeks, especially preterm neonates. Monitor liver tests; if abnormalities occur consider discontinuation or dosage reduction.
Hypersensitivity Reactions: Monitor for signs or symptoms. Discontinue infusion if reactions occur.
Risk of Infections, Fat Overload Syndrome, Refeeding Syndrome, Hypertriglyceridemia, and Essential Fatty Acid Deficiency: Monitor for signs and symptoms; monitor laboratory parameters.
Aluminum Toxicity: Increased risk in patients with renal impairment, including preterm neonates.
Most common adverse drug reactions (≥5%) from clinical trials in adults were nausea, vomiting, and hyperglycemia. Most common adverse drug reactions (≥5%) from clinical trials in pediatric patients were anemia, vomiting, increased gamma-glutamyltransferase, and nosocomial infection.
To report SUSPECTED ADVERSE REACTIONS, contact Fresenius Kabi USA, LLC at 1-800-551-7176, option 5, or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
This Important Safety Information does not include all the information needed to use SMOFlipid safely and effectively. Please see full prescribing information, for intravenous use at www.FreseniusKabiNutrition.com/SMOFlipidPI.
*Data on file.

