Kabiven’s 3-chamber bag
simplifies PN
Learn about the features of our unique
3-chamber bag and how to activate it.
Learn about the features of the Kabiven three-chamber bag and how to activate it. Watch the video.

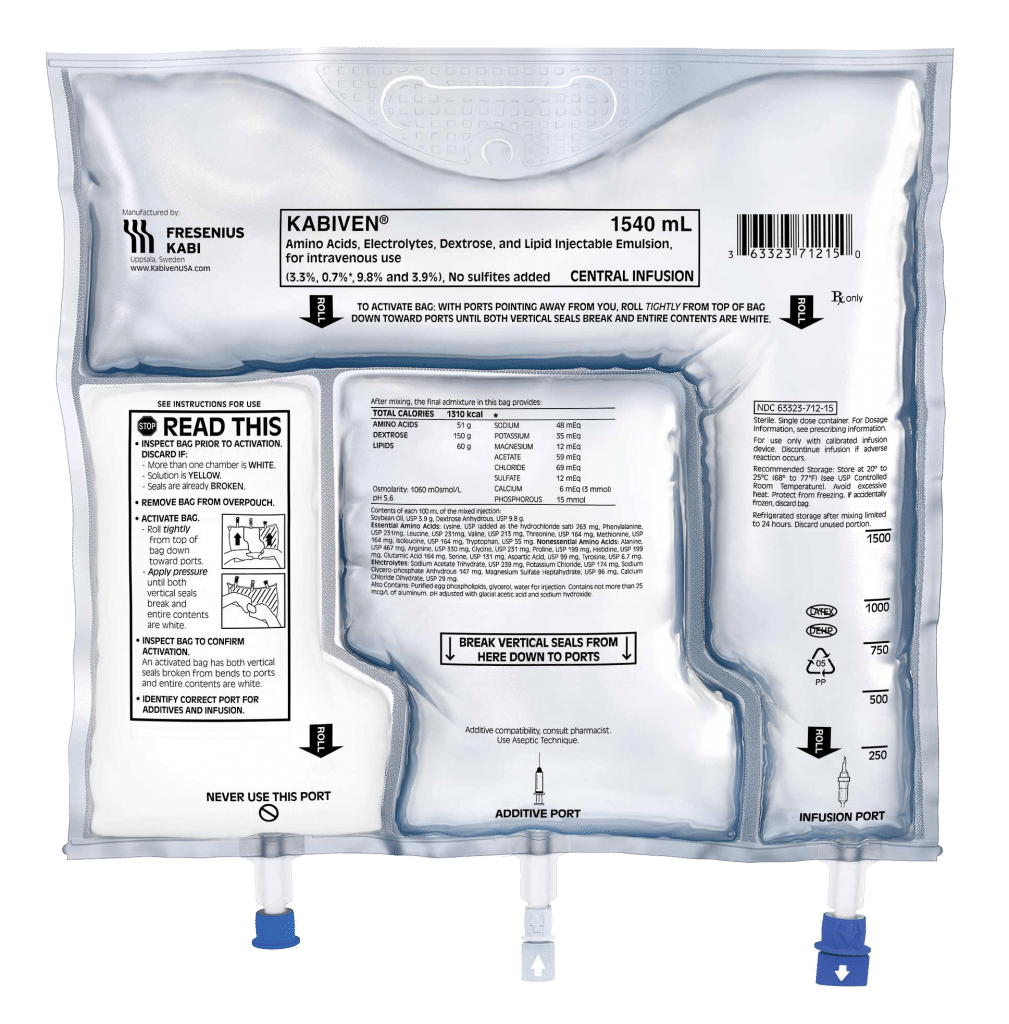
Kabiven/Perikabiven’s unique design streamlines the delivery of nutrition therapy to patients by simplifying calculations, prescription writing, compounding, and administration, all while supporting PN safety by minimizing the risk of contamination.1 The Kabiven and Perikabiven 3-chamber PN bags efficiently deliver 3 macronutrients (dextrose, protein, and lipids) plus electrolytes in volumes and concentrations that meet the needs of most PN patients.2,3
Kabiven: Full prescribing information>
An all-in-one solution in a 3-chamber bag can simplify:

Calculations for dietitians

Compounding for pharmacists

Prescription writing for clinicians

Administration for nurses
Learn about the features of our unique
3-chamber bag and how to activate it.
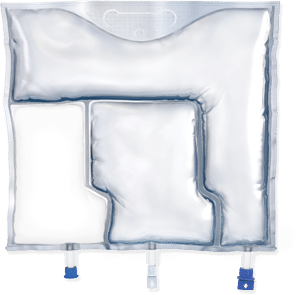

Fresenius Kabi pioneered the use of an all-in-one, 3-chamber bag designed for the efficient delivery of PN.
Indication:
Kabiven and Perikabiven are each indicated as a source of calories, protein, electrolytes, and essential fatty acids for adult patients requiring PN when oral or enteral nutrition is not possible, insufficient, or contraindicated. Kabiven and Perikabiven may be used to prevent essential fatty acid deficiency or treat negative nitrogen balance in adult patients.2,3
Kabiven is indicated for intravenous infusion into a central vein.2 Perikabiven is indicated for intravenous infusion into a peripheral or central vein.3
Limitations of Use:
Neither Kabiven nor Perikabiven are recommended for use in pediatric patients <2 years, including preterm infants because the fixed content of the formulations do not meet the nutritional requirements in this age group.2,3
Fresenius Kabi’s patient support program, KabiCare, offers online resources for claims appeals and billing as well as coding guides for parenteral nutrition.
Explore additional Kabiven/Perikabiven materials in our Resource Center.
For Consumers
KABIVEN (Amino Acids, Electrolytes, Dextrose, and Lipid Injectable Emulsion), for intravenous use
PERIKABIVEN (Amino Acids, Electrolytes, Dextrose, and Lipid Injectable Emulsion), for intravenous use
IMPORTANT SAFETY INFORMATION
What is Kabiven and Perikabiven?
Limitations of Use
Neither Kabiven nor Perikabiven is recommended in pediatric patients less than 2 years old because the fixed amount of the formulations do not meet nutritional needs in this age group.
Do not use Kabiven or Perikabiven in patients who have:
For Consumers
KABIVEN (Amino Acids, Electrolytes, Dextrose, and Lipid Injectable Emulsion), for intravenous use
PERIKABIVEN (Amino Acids, Electrolytes, Dextrose, and Lipid Injectable Emulsion), for intravenous use
IMPORTANT SAFETY INFORMATION
What is Kabiven and Perikabiven?
Limitations of Use
Neither Kabiven nor Perikabiven is recommended in pediatric patients less than 2 years old because the fixed amount of the formulations do not meet nutritional needs in this age group.
Do not use Kabiven or Perikabiven in patients who have:
References: 1. Derenski K. Standardize parenteral nutrition using commercial premix. Pharm Purchasing Prod. 2012;1-7. 2. Kabiven Prescribing Information, Fresenius Kabi USA, LLC. 2023. 3. Perikabiven Prescribing Information, Fresenius Kabi USA, LLC. 2023. 4. Ayers P, Adams S, Boullata J, et al. A.S.P.E.N. parenteral nutrition safety consensus recommendations. JPEN Parenter Enteral Nutr. 2014;38:296-333.